Best Clinical Research Management Software
As the world of clinical research continues to evolve, it is essential to handle complex data, follow compliance regulations, and ensure the efficient communication of stakeholders in clinical trials. Clinical Research Management Software (CRMS) provides the tools to tackle these issues, with the ability to improve productivity, precision, and integrity of the research process. In this blog, we will explore the best clinical research management software available, their key features, advantages, and the significance of selecting the appropriate solution for clinical research.
What is Clinical Research Management Software?
Clinical Research Management Software (CRMS) is a highly specialized tool that was created to assist with the management of clinical trials. It offers solutions for almost all the processes involved in clinical trials, such as subject recruitment, data management, regulatory oversight, and reporting in research organizations, pharmaceutical companies, and Cro (Contract Research Organizations).
A robust CRMS system facilitates smoother project management, improves data quality, ensures regulatory compliance, and ultimately accelerates the time to market for new medical treatments.
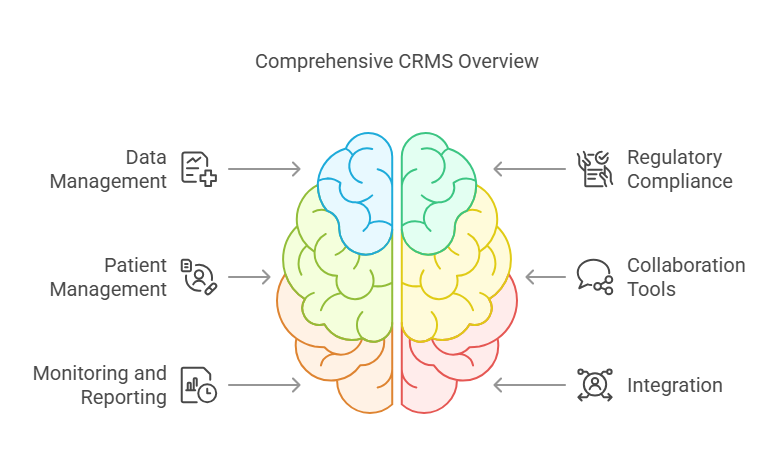
Key Features of CRMS:
Data Management: Organize, store, and analyze clinical trial data efficiently.
Regulatory Compliance: Ensure adherence to guidelines such as ICH-GCP, FDA, and HIPAA.
Patient Management: Handle recruitment, consent, and tracking of patient progress.
Collaboration Tools: Streamline communication between researchers, sponsors, and clinical sites.
Monitoring and Reporting: Track trial progress in real-time and generate accurate reports.
Integration: Easily integrate with other healthcare and data systems, such as Electronic Data Capture (EDC) systems.
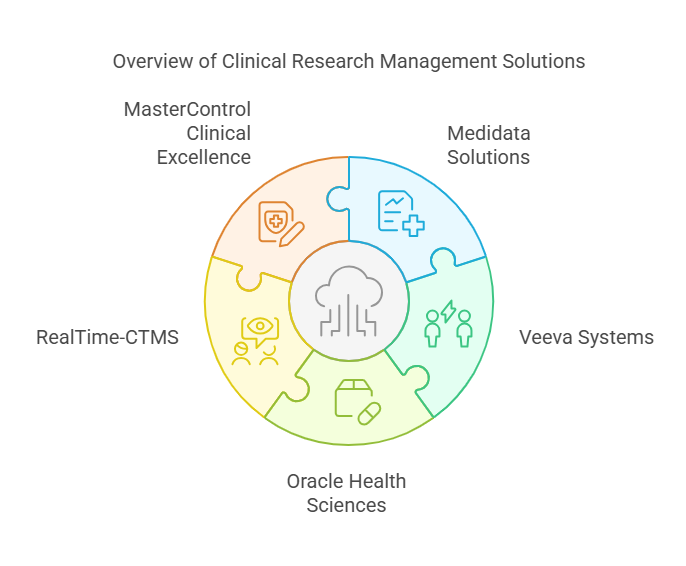
Top Clinical Research Management Software Solutions
Here, we will explore some of the best clinical research management software solutions available today. These platforms are renowned for their efficiency, adaptability, and ability to enhance clinical trial outcomes.
1. Medidata Solutions
Medidata Solutions is a leading CRMS platforms, which has a rich feature set to address each phase of the clinical trial. Medidata's cloud based system is used by research professionals to design the trial, recruit patients and manage the data. Its Rave EDC system provides an easy to use interface for data capture, so that data from trials is accurate and secure.
Key Features:
Advanced data analytics and AI capabilities
Comprehensive data management and monitoring tools
Seamless integration with other clinical systems
Regulatory compliance support
Why Choose Medidata?
For organisations that need a solution that can easily scale up to an enterprise level and support precise management of global clinical trials, Medidata is ideal. It's advanced analytics features help data inform decisions that reduce trial costs and time lines.
2. Veeva Systems
Veeva Systems is a favorite among clinical research organizations because of its commitment to innovation and compliance. Veeva's Vault Clinical Suite is built to improve the efficiency of clinical trial management with solutions for study coordination, monitoring, and reporting. The software is cloud-based for easy access and collaboration among many stakeholders.
Key Features:
Cloud-based access for real-time collaboration
Integrated data capture and management
Customizable workflows for different trial phases
Robust reporting tools for compliance and audit readiness
Why Choose Veeva Systems?
For organisations that want to streamline their clinical operations without compromising on compliance to regulatory standards, Veeva’s Vault Clinical Suite has been designed. It is ideal for multi-site trials and flexible platform requirement that are a fit for such organizations.
3. Oracle Health Sciences
Oracle Health Sciences provides an integrated solution for managing the clinical supply chain from planning to execution and delivery, with end-to-end visibility and control. The platform streamlines operations with automated processes and provides real-time data for better decision-making, ultimately reducing costs and improving patient care. The CTMS solution provided by Oracle Health Sciences aims to enhance the overall effectiveness of trial execution. Oracle's CRMS offers features for patient enrollment, site assessment, and study document management, thus offering end-to-end solutions for managing complex clinical trials.
Key Features:
Integrated site and patient management
Real-time data tracking and analytics
Supports multi-site and global clinical trials
Robust audit trail for compliance
Why Choose Oracle Health Sciences?
For instance, organizations that are involved in big and complicated clinical trials that need strong data security, online reporting, and strong compliance features, Oracle is ideal.
4. RealTime-CTMS
RealTime-CTMS is a very easy to use CRMS solution for clinical trial sites and organizations. It provides solutions for all aspects of clinical trials management right from patient recruitment, budgeting to regulatory compliance.
Key Features:
Customizable workflows to suit site-specific needs
Real-time patient tracking and communication tools
Budget and finance management capabilities
Regulatory compliance support
Why Choose RealTime-CTMS?
This platform for small research sites and of organizations that need a affordable but powerful tool for patient management and site operations.
5. MasterControl Clinical Excellence
Through MasterControl Clinical Excellence, organizations can combine document control, training, and clinical trial management in one single platform. The focus is on compliance with FDA and other regulatory bodies in the conduct of clinical trials efficiency.
Key Features:
Comprehensive document and workflow management
Training and compliance tracking
Integrated patient and site management tools
Strong regulatory compliance features
Why Choose MasterControl?
For organizations seeking to simplify their clinical trials by integrating document management with CRMS, MasterControl is the solution. It helps ensure compliance and operational efficiency by streamlining the process.
How to Choose the Best Clinical Research Management Software?
Selecting the right clinical research management software depends on your organization’s unique needs, the scale of the trials, and the specific features that will help you meet regulatory and operational goals. Here are key factors to consider when choosing the best CRMS for your organization:
1. Scalability
Clinical trials can vary in size, from small-scale studies to global, multi-site trials. Ensure that the CRMS you choose can scale with your organization’s needs, handling everything from patient recruitment to data management and reporting.
2. User-Friendliness
A CRMS should be intuitive and easy to use for all stakeholders involved in the trial, including researchers, clinical staff, and sponsors. A user-friendly interface reduces the time spent on training and minimizes the chances of user errors.
3. Compliance Features
Regulatory compliance is one of the most critical aspects of clinical research. The software you choose should support compliance with FDA, ICH-GCP, and HIPAA regulations, ensuring that your trials meet all necessary legal and ethical standards.
4. Integration Capabilities
A robust CRMS should integrate with your existing systems, such as EDC, Electronic Medical Records (EMR), and Laboratory Information Management Systems (LIMS). This ensures a seamless flow of data and reduces the need for manual data entry, which can introduce errors.
5. Cost and Budgeting
The cost of CRMS platforms can vary widely. It’s essential to balance the features you need with your budget, ensuring that you’re not paying for functionalities that won’t benefit your trial operations. Some software solutions offer flexible pricing models, such as subscription-based or usage-based pricing, making it easier to fit into your budget.
6. Data Security
Clinical trials involve sensitive patient data, making data security a top priority. Choose a CRMS that offers robust encryption and security measures to protect data and ensure compliance with privacy regulations like GDPR and HIPAA.
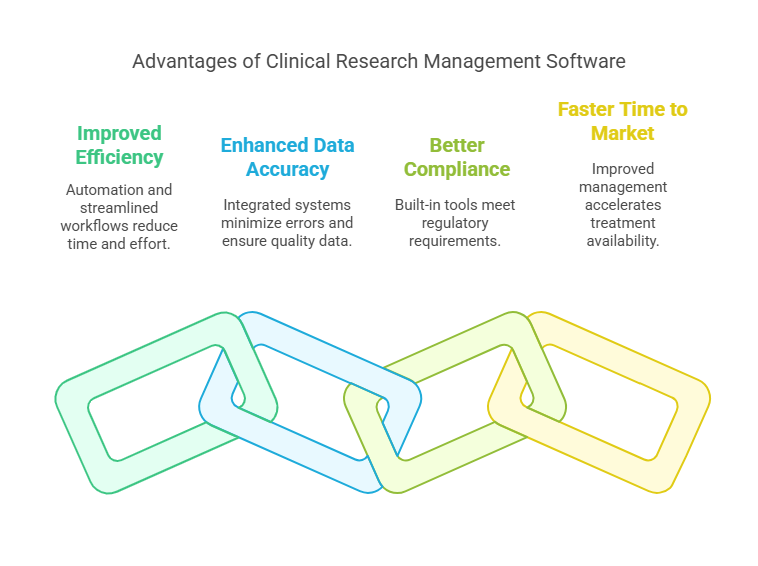
Benefits of Using the Best Clinical Research Management Software
Implementing a high-quality clinical research management system brings numerous benefits, including:
Improved Efficiency: Automation of repetitive tasks and streamlined workflows reduce the time and effort required to manage trials.
Enhanced Data Accuracy: Integrated data management systems reduce the likelihood of errors and ensure high-quality data for analysis.
Better Compliance: Built-in compliance tools ensure that your trials meet all necessary regulatory requirements.
Faster Time to Market: Improved project management and data analysis capabilities accelerate the process of bringing new treatments to market.
Conclusion
Choosing the best clinical research management software is critical for the success of clinical trials. The right solution can streamline operations, ensure regulatory compliance, and enhance data accuracy, ultimately accelerating the development of new medical treatments. Whether you’re managing small-scale studies or large, multi-site global trials, investing in the right CRMS will help your organization conduct more efficient and successful trials.
To explore additional training on clinical trial management and learn more about optimizing your clinical research processes, consider enrolling in one of our courses, such as the Clinical Trials Assistant Training.
For more in-depth resources on clinical research management, you can visit authoritative sources like those provided by academic institutions such as Johns Hopkins University and Harvard University.
Course Links:
Reference Links:
Harvard Catalyst - Clinical Research Resources - Offers extensive resources for managing clinical trials and research practices.
Society of Clinical Research Associates (SOCRA) - Provides certifications and education related to clinical research roles, including CRMS platforms.
National Institutes of Health - Clinical Trials - Information on clinical trials, management practices, and tools to enhance trial success.