The Role and Importance of Clinical Research Coordinators
The healthcare and pharmaceutical industries depend heavily on Clinical research coordinators (CRCs). CRCs oversee clinical trials to guarantee both regulatory compliance and scientific protocol execution. CRCs act as connectors between patients and healthcare providers and researchers while maintaining stakeholder communication. This blog examines CRC responsibilities and qualifications and career opportunities with an emphasis on their salary structure at Massachusetts General Hospital and other similar institutions.
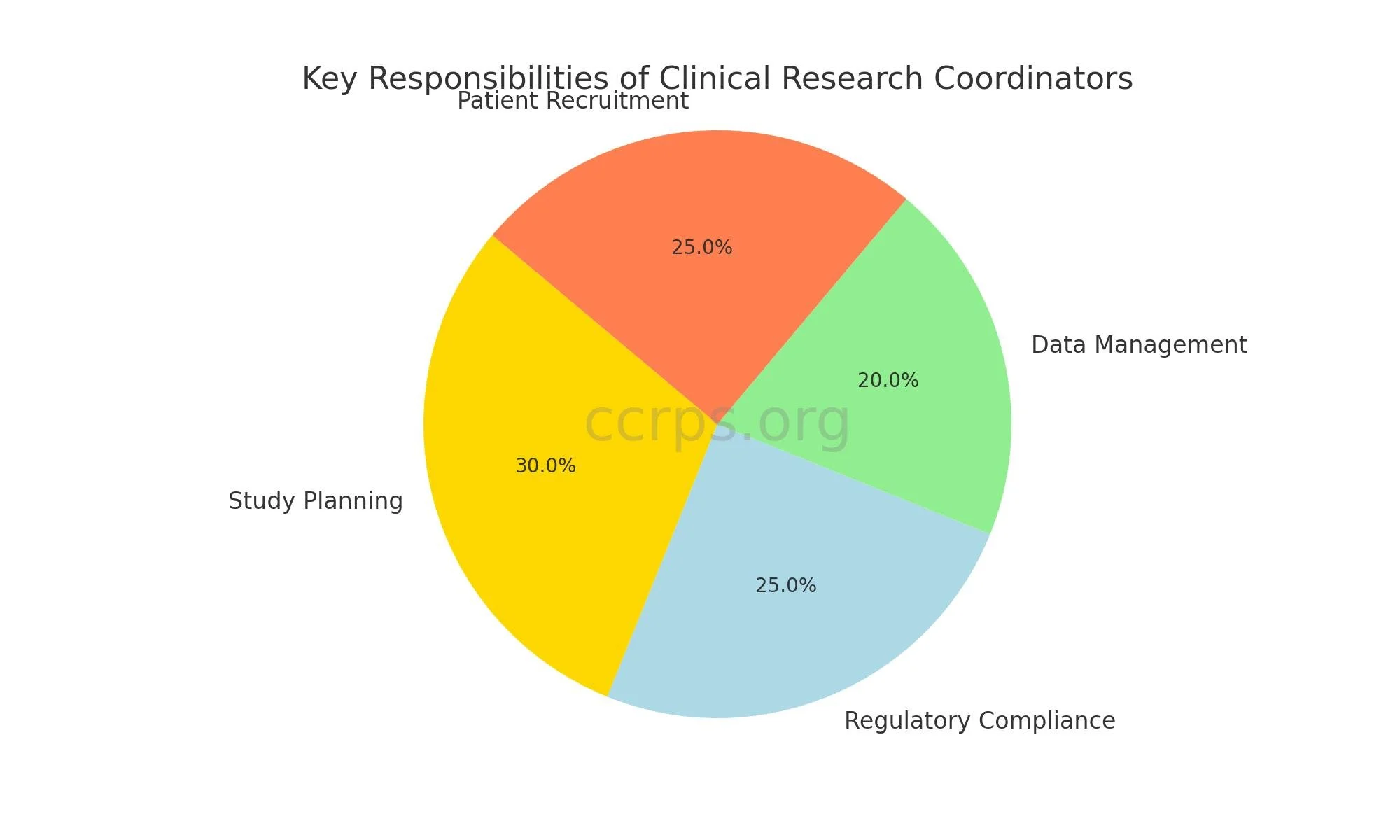
Responsibilities of Clinical Research Coordinators
Clinical research coordinators execute multiple responsibilities to guarantee clinical trials operate efficiently and smoothly. Their responsibilities include:
Study Planning and Coordination: CRCs help in the development of study protocols, time management of the study and coordination of different trial stages.
Regulatory Compliance: They guarantee that all trials are in conformity with the regulatory requirements including making sure that all necessary approvals from Institutional Review Boards (IRBs) are obtained.
Data Management: CRCs perform data collection tasks along with data entry and maintenance duties to guarantee both data integrity and confidentiality.
Patient Recruitment and Communication: They recruit and screen potential study participants, provide informed consent, and maintain ongoing communication with participants throughout the study.
References
Qualifications and Skills Required
To become a clinical research coordinator, certain educational and professional qualifications are typically required:
Educational Background: A bachelor's degree in life sciences, nursing, or a related field is usually required. Advanced degrees or certifications can be advantageous.
Certifications: Professional certifications such as the Certified Clinical Research Coordinator (CCRC) can enhance job prospects and credibility.
Skills: Essential skills include strong organizational abilities, attention to detail, excellent communication skills, and proficiency in using clinical trial management software (CTMS).
Career Prospects and Salary
The career prospects for clinical research coordinators are promising, with a growing demand for clinical trials. Salaries can vary widely based on experience, education, and location. Clinical research coordinators at Massachusetts General Hospital receive competitive salaries because of the institution's high standing.
Salary Insights at Massachusetts General Hospital
Massachusetts General Hospital follows a salary structure for clinical research coordinators that depends on their qualifications and experience. The average annual salary for CRCs at Massachusetts General Hospital stands at $60,000 while experienced staff members can earn more based on their qualifications.
Reference
The Impact of Clinical Research Coordinators
Clinical research coordinators play a pivotal role in advancing medical research and improving patient care. Their contributions ensure the successful implementation of clinical trials, leading to the development of new treatments and therapies.
Key Contributions
Advancing Medical Knowledge: By managing clinical trials, CRCs contribute to the generation of new medical knowledge and innovations.
Ensuring Patient Safety: They ensure that trials are conducted safely and ethically, prioritizing patient welfare.
Facilitating Regulatory Approval: CRCs help navigate the complex regulatory landscape, facilitating the approval of new drugs and treatments.
References
Challenges Faced by Clinical Research Coordinators
Despite the rewarding nature of their work, clinical research coordinators face several challenges:
Regulatory Hurdles: Navigating complex regulatory requirements can be challenging and time-consuming.
Participant Recruitment: Recruiting and retaining study participants can be difficult, impacting study timelines.
Data Management: Ensuring the accuracy and confidentiality of clinical trial data is crucial and demanding.
References
Enhancing the Role of Clinical Research Coordinators
To address these challenges and enhance the role of CRCs, several strategies can be implemented:
Training and Education: Continuous training and education programs can help CRCs stay updated with regulatory changes and new methodologies.
Technology Integration: Utilizing advanced clinical trial management systems can streamline data management and improve efficiency.
Collaboration and Support: Encouraging collaboration among stakeholders and providing adequate support can alleviate some of the challenges CRCs face.
Future Trends in Clinical Research Coordination
The field of clinical research coordination is evolving, with several trends shaping its future:
Increased Use of Technology: Technology, including artificial intelligence and machine learning, is increasingly being integrated into clinical trials to enhance efficiency and accuracy.
Focus on Patient-Centered Trials: There is a growing emphasis on patient-centered approaches, ensuring that trials are designed with the patient experience in mind.
Global Collaboration: International collaboration is becoming more common, enabling more extensive and diverse clinical trials.
References
Conclusion
Clinical research coordinators are integral to the success of clinical trials, playing a vital role in advancing medical research and improving patient care. Their contributions, challenges, and future prospects highlight the importance of this profession. As the field continues to evolve, CRCs will remain at the forefront of medical innovation, ensuring the safe and effective conduct of clinical trials. For those looking to elevate their expertise and career prospects in this field, obtaining certification from the CCRPS can provide a valuable credential, further enhancing their qualifications and opportunities within the industry.
Explore Courses for Clinical Research Career
Courses Available: