Clinical Research Associate Job Description
Read more about the Clinical Research Associate Jobs descriptions and skills that require in Clinical Research Associate post. Visit us now!
Clinical research associates (CRAs) are responsible for organizing and administering clinical research trials for new or current drugs so that they may assess the risks and benefits of using them. The major responsibilities of clinical research associate jobs are:
They write out the procedures for administering drug trials.
They identify and brief appropriate trial investigators.
They make use of their knowledge of the basics of clinical research to collect and analyze and record data.
They design trail materials and supply the research study centers with the quantities that they need.
They monitor the process of the trial from the start to the finish.
Clinical research associates are always present in the institute of clinical research because of the essential duties that they perform. However, the position is very competitive according to various job websites. As competitive as it is, it is also lucrative. Clinical research associate can work in government clinical research institute, pharmaceutical companies, private owned clinical research institute, etc. Their salaries may differ based on how much the individual companies or employees offer at their different choice of work place. But on the average, clinical research associates are paid around $65,000 per year. This leaves the pay at about $24 - $25 per hour.
There are various factors that can affect how much a clinical research associate (CRA) goes home with at the end of the month or cumulatively at the end of the year. These factors can be the location of the company, that is, the economic strength of the country or city where they are working, the degree or certificate or the level of education and exposure that the CRA has, the number of years if working experience that they have accumulated. These factors work together to determine how much they take home.
You can boost your chances of getting a higher pay, in the region of $85,000 to $90,000 or even more by getting the necessary education and certification. For example, experienced contract CRAs can earn up to $300k.
Skills required to be successful as a clinical research associates include:
A logical and inquisitive mindset with quick thinking.
Good organizational abilities.
Excellent communication skills. Both written and verbal are important.
Commercial awareness.
Confidence
The basic requirement to be a clinical research associate is an undergraduate degree or a postgraduate degree in related life sciences fields (like biology, toxicology, microbiology, pharmacology, biochemistry), nursing, or medical sciences (like immunology, pharmacy, anatomy, physiology, or medicine). Due to the competitive nature of the job, some people go ahead and obtain a doctorate degree in any of the above mentioned fields. This gives them a better chance of getting a senior level job and increase their promotion prospect.
Beyond academic degrees, you need to be certified to become a clinical research associate. You need certification from organizations like ACRP (association of clinical research professionals) or SOCRA (society of clinical research associates). You will need to obtain certification course and pass the exams to become certified. At ccrps.org, we offer ACCRE accredited courses and insider information on everything you need to know for a career in clinical research. You can click here to see the offers for the courses available.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Clinical Research Opportunities in New York City
Clinical research in New York has been on the rise in recent years. New York City is known for its advancement in clinical research and management.
Participating in a clinical research in New York is beneficial to the participants and it is a way for you as the participant to help people with such health issues and also contribute to the medical sciences. Popular benefits include:
Getting free medical care.
Being involved actively.
It helps you to improve your lifestyle for better health.
You have access to and benefit from new treatments that are not yet available for the general public.
Clinical research in US have been ongoing for a long time. With every discovery and advancement, it becomes more important part of healthcare. If you are interested in being a clinical researcher in US, you can get all the help that you will need to be successful at CCRPS. From knowledge to experience to degree and certification, you will get all the needed help from experts and professionals in the clinical research industry that are willing and ready to set you on the right path.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Clinical Research Field Knowledge Everyone Should Know
What is Clinical Research?
Clinical research is a branch under healthcare sciences that is responsible for finding out the effectiveness and safety of medical devices, medications, diagnostic products and treatment regimens that are intended for human use in the cure of diseases and illnesses.
Clinical research is used for the diagnosis, prevention, treatment, relieving or alleviating of symptoms of a disease, and is much different from clinical practice. The major difference between clinical practice and clinical research is this - clinical practice works with only established treatments, while clinical research works to make a new established treatment.
Clinical research spans from inception tests to introduction to the consumer market. It goes through stages of tests called phases. It is done with the main purpose of testing the effectiveness and safety of a drug for human use.
Bodies Concerned With Clinical Research
Europe
In the European countries, the European Medicines Agency (EMA) is the regulatory body that facilitates clinical research studies. Human subjects that have given their informed consent are allowed to participate as research subjects in the 4 phases of clinical research.
United States
In the United States, the conduction of clinical research is a little more complex.
A new drug or treatment that has not yet been approved or cleared by the Food and Drug Administration (FDA) cannot be given a license. Clinical research data has to be submitted to the FDA through the support of an (IND) Investigational New Drug application. This enables the FDA to review the new treatment before they are used to conduct studies involving human subjects. After all proper investigations by the FDA, the new drug or treatment can now be licensed and advertised.
In the case of medical devices, if the device shows signs of significant risk or has not been exempted or licensed by the FDA, then the clinical research data has to be submitted to the FDA through the support of an (IDE) Investigational Device Exemption application. This process may also require approval from any of the following; Institutional committee reviews, Radiation Safety Committee, Conflict of Interest Committee, Privacy Board, Radioactive Drug Research Committee, Research Ethics Board (REB) or the Institutional Review Board (IRB).
Criteria for Review
The criteria for the review of all clinical research depends on the following;
• Federal regulations that the research falls under and is subjected to.
• The regulations the institutions subscribe to.
• The response to local, state, federal laws, policies, rules, regulations, or accreditation entity recommendations.
The last criteria for review is supervised by the IRB or ERB in concordance with the regulatory affairs to protect human subjects.
Where Do Clinical Researches Take Place?
Clinical researches usually take place at affiliated research study sites, hospitals, healthcare clinics, or academic medical centers. These places are strategically placed to provide the competence of the academic institutions involved as well as provide closeness to the medical participants pool.
The ethical issues and conducts of clinical research is overseeing and supervised by their internal institutional review boards.
There are lots of professionals involved in the world of clinical research, as it is a complex network of pharmaceutical companies, academic research institutions, and biotechnology companies. All these has led to the fast growth of technologies used in the operational factors and management aspects of clinical research.
The operational factors are overseen by clinical research professionals such as, CRAs, CTAs, CRCs, and others, while the management aspects of clinical research are overseen by CTMs, CRMs, CRPMs, CrDMs, and others.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
How To Maximize Using The Clinical Research Wiki
One way you can be sure to get answers to all of your clinical research questions is going through the clinical research wiki. All clinical research information that pertains to important issues such as the different fields of clinical research, the roles and functions of all the different clinical research professions, how to work in clinical research are available on this site.
For example, let’s say you want to know how to define clinical research. According to the wiki, clinical research is defined as a branch of healthcare science that determines the safety and effectiveness (efficacy) of medications, devices, diagnostic products and treatment regimens intended for human use.
With this definition from wiki, it is easy to see why clinical research is very important to medical science and the healthcare system. However, it is important to know that because the wiki can be changed and altered, it is important to double check any information by looking at the source material sited at the bottom of the page.
Once you know how to utilize a search engine like the clinical research wiki, you can learn so much about the various aspects of the field. This is especially important if you are looking to start out a career in clinical research.
Whether you are starting out as a clinical research assistant, a clinical research associate, or a clinical research coordinator; all of them have basic requirements that you need to meet. It could be a degree or passing certification. Chances are, you will need help passing the certificate examinations conducted by the Association of Clinical Research Professionals. That is where CCRPS comes in. CCRPS provides quality clinical research programs and will equip you with all you need to have a successful career.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
How to Get into Clinical Research Step by Step
Looking for clinical research jobs? Visit CCRPS at here you can get the all information about clinical research remote jobs. Apply now!
What is Clinical Research?
Clinical research is a branch under the healthcare sciences that is responsible for finding out the effectiveness and safety of medical devices, medications, diagnostic products and treatment regimens that are intended for human use to help cure illnesses. Clinical research is important for the diagnosis, prevention, treatment, alleviating of symptoms of a disease, but it is much different from clinical practice.
You want to get a career for yourself in clinical research, then let's take you through the drill.
The first step is to get an education.
Earn a bachelor's degree in a life science or health related discipline. Courses like medicine, pharmacology, biology, molecular biology, genetics, anatomy, biotechnology, nursing, physiology, chemistry, or bioengineering will equip you with the necessary and relevant knowledge to get you into clinical research.
Supplement your education by applying for the courses offered at your university or from professional organizations like Certified Clinical Research Professionals (CCRPS) and Association of Clinical Research Professionals (ACRP). These courses will include; study designs, Good Clinical Practices (GCP), research ethics, drug development cycle, regulatory affairs and requirements both in the US and internationally, among others.
Get a certification from a reputable organization, such as CCRPS, ACRPS, and Society of Clinical Research Associates (SOCRA).
Make sure you study the ICH GCP guidelines and ethics to make you competent. Training in the International Conference on Harmonisation (ICH) Good Clinical Practices (GCP) ethics and guidelines improves your chances greatly. At CCRPS, we offer a free ICH GCP certification to help you get started.
Keep proper documented records of your certifications as well as your education.This will save you a lot of time and stress.
The next step is to gather experience.
The following are ways you can gather experience:
Volunteer - Look for volunteering opportunities around your area and volunteer to help with the projects that will be carried out in the clinical research industry. This helps you get closer to the professionals as well as the tasks itself. You can volunteer at clinical research professionals organizations related to the clinical research field or medical field, medical centers or hospitals, International Review Boards (IRB) or Research Ethics Committees.
Research Projects - Take up clinical research monitoring projects and you can gain at least two years of experience.
Internships - Seek out an internship with medical firms, biotechnology companies, and pharmaceutical companies while you're still in college. CCRPS offers an internship program that can help you develop your resume.
The next step after getting an education and gathering experience is to apply for entry-level positions
A job as a clinical research professional is a rewarding career, and as such you must not only get an education and experience, but you must also be able to put in a high quality CV and cover letter for your application.
As long as you have a bachelor's degree and at least one year of experience in clinical research, you can easily qualify for an entry level position in clinical research. A quick search for “clinical research jobs near me” allows you gain more insight into the clinical research industry.
So, how do you get a job?
Here are tips that you need to keep in mind when you are applying for clinical research jobs.
• Be realistic about the jobs you can go into with your educational background and experience. Apply for entry-level positions firsts, then work your way to the top with targeted efforts and tenacity.
• Read each job descriptions clearly and highlight on your CV the relevant experience that you have that matches the specific role requirements. That little edit can change the way clinical research recruiters and consultants look at your CV as compared to others.
• Network with hiring managers and clinical research recruiters to expand your base as not all companies will advertise their vacancies. Upload your CV to a database that enables employers, clinical research recruiters, and hiring managers to easily find you.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Clinical Research Coordinator Jobs
Looking for clinical research coordinator jobs? Visit CCRPS and check out the recent job post descriptions and skills required for CRC.
Clinical research coordinators have a responsibility to administer clinical trials. Their duty apart from this also include collecting data, giving out questionnaires and informing study participants about the aims and objectives of the study.
Their overall duty are as follow:
They oversee the smooth running of clinical trials in the clinical research facility
They collect, code and analyze data gotten from the research.
They manage the research budget.
They carefully monitor the clinical trials to endure that they comply with moral, ethical and regulator standards.
They are actively involved in the recruitment of subjects for the trials.
They make sure that needed supplies and equipment are available and ready for use.
As a clinical research professional, clinical research coordinators work with and directly under the clinical research project manager. The work of the CRC is actually a very important one in any clinical research institute. There are many clinical research job websites that have laid down requirements for the post of the clinical research coordinator. These requirements could include:
An associate degree in nursing or any other related fields.
Should have at least 2 years experience in healthcare.
Very good interpersonal skill.
An ability to communicate effectively in both written and oral modes.
Must have an analytical mindset and excellent organizational skills.
Must be ready to learn and self-educate while at the job.
The clinical research coordinators understand the basics of clinical research and are expected to put this knowledge into play when organizing their team of clinical research associates and the research lab as well.
Much has been said about the work of a clinical research coordinator but no one becomes a CRC in a day. There are degrees, academic requirements certificates that you must possess before you can become or offer a job as a clinical research coordinator. You will need certification from the ACRP (Association of Clinical Research Professionals) or SOCRA (Society Of Clinical Research Associates). Getting the required certificate will mean that you have fulfilled the eligibility and have passed the necessary examinations. This can be quite a task, but at ccrps.org, we make passing the examinations and getting the certificates easier for you. You can register for our available courses and get the help needed to take your clinical research coordinator career to the next level.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Clinical Research Project Manager
Clinical Research Project Manager - A Full Guide To Becoming a Clinical Trial Project Manager
As of mid-April 2022, top recruitment platform ‘indeed.com’ lists 6,026 openings for Clinical Research Project Managers across the United States[1]. According to ‘payscale.com’, remuneration for this profile ranges from $84,168 for early-career to $120,501 for experienced Clinical Research Project Managers, with a mid-career median of $107,649[2].
A Clinical Research Project Manager (henceforth CRPM) is also known as a Clinical Research Manager or a Clinical Trial Manager. The role of a CRPM is to provide leadership during the clinical trial of new drugs, with the overarching goal of ensuring that the clinical trials process is completed within the stipulated time-frame and allocated budget, while maintaining the highest standards of research quality and scientific integrity.
An overview of the CRPM profile
A Clinical Research Project Manager is the liaison between the study sponsor and the clinical research team. Thus, the CRPM must balance the expectations of the funding organization in a clinical research study (pharmaceutical company, governmental agency, university research division or other entity) with the needs of the team, including Principal Investigators (PIs) and co-PIs, Clinical Research Coordinators (CRCs), Clinical Research Associates (CRAs), Clinical Trial Assistants (CTAs) and other site staff.
The CRPM plays a leading role in planning, implementation and ongoing monitoring of live clinical trials. In the process, a CRPM also mentors junior team members, including CRAs and CTAs.
Evidently, a career as a CRPM is rewarding, but also demanding. So, what does it take to qualify and be recruited as a Clinical Research Project Manager?
The road-map to becoming a CRPM
Even if you’re new to the field of clinical research, you’ve probably guessed by now that becoming a Clinical Research Project Manager takes both qualifications and experience. A typical CRPM has a bachelor’s (or master’s) degree in life science, combined with four to five years’ experience in one or more clinical research roles such as CRC, senior CRA or QPPV (Qualified Professional in Pharmacovigilance).
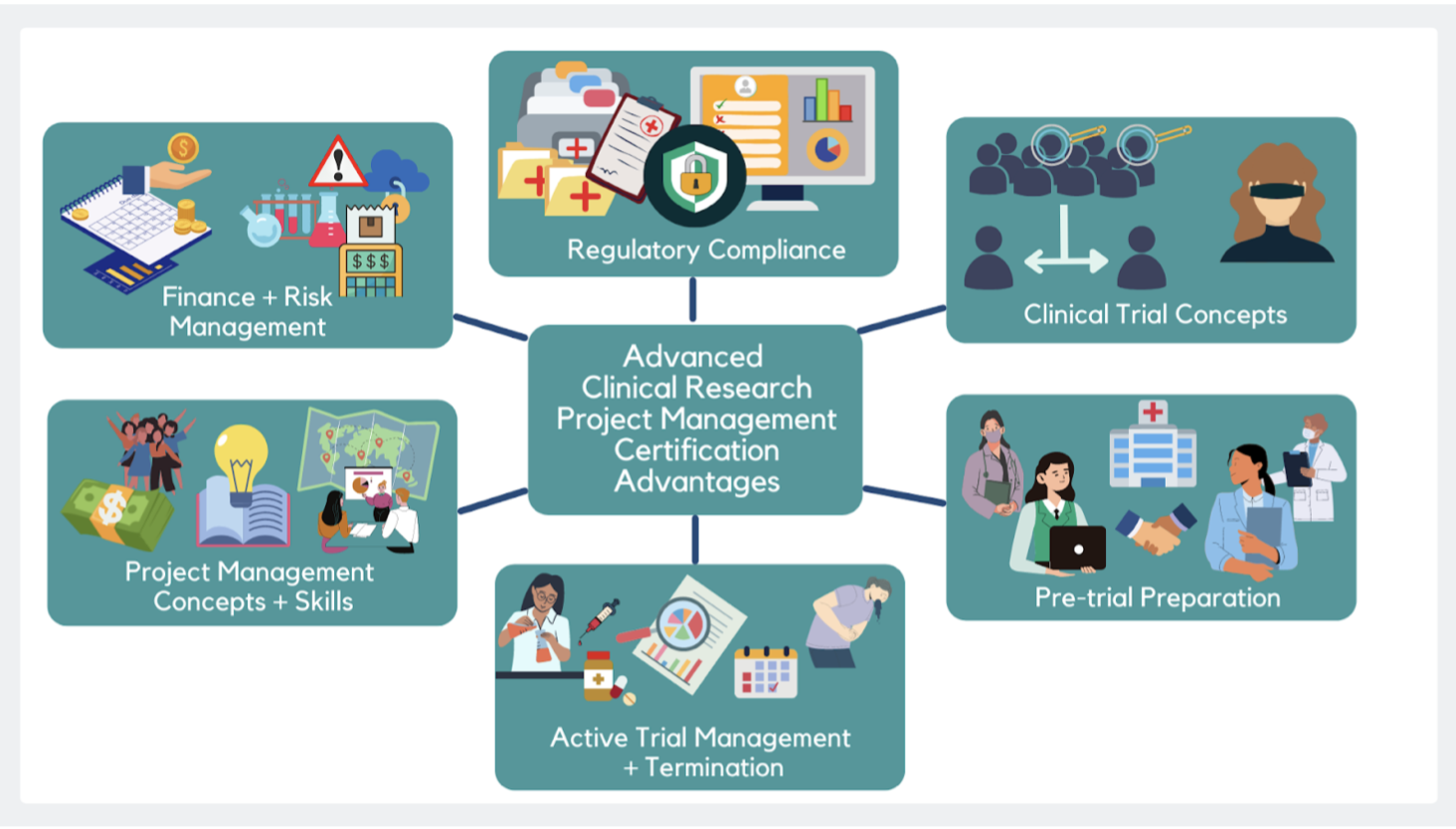
Irrespective of where you are along this path, the remainder of this article charts a clinical research professional’s journey of growth in developing into a competent CRPM. For conceptual convenience, the process is discussed in two parts – Part 1 centers on the research and administrative skills needed for a CRPM, while Part 2 revolves around expertise in project management. The final sections address how the Advanced Clinical Research Project Manager Certification or ACRPMC facilitates the transition to a CRPM role.
The CRPM journey, Part 1: Research and administrative skills
By the time a clinical research professional is eligible to apply for a CRPM position, s/he has typically developed a range of competencies in the day-to-day running of clinical trials. In addition, a professional at this stage has usually acquired a good working knowledge of the planning and oversight required for a clinical research study.
In broad strokes, a CRPM candidate likely already has a knowledge base and skill sets in the following areas:
1. ICH-GCP guidelines, Title 21 of FDA CFR and IRB compliance
One of the first things every clinical research professional learns is the importance of adhering to the principles and policies of good clinical practice, as outlined in the ICH-GCP[3]—these guidelines set the framework for carrying out research in a manner that prioritizes the safety and welfare of human subjects and the quality and integrity of clinical data.
The ICH-GCP principles are mirrored by Title 21 within the US FDA’s Code of Federal Regulations (CFR)[4]. Clinical research across the US must conform to the requirements of Title 21. Part of Title 21 regulatory compliance requires nearly all clinical research studies to first be approved by an Institutional Review Board or IRB (also called an Ethics Committee). As clinical trials progress, any changes to the study trial protocol, consent procedures or data management system must likewise first be reviewed and approved by the IRB.
The CRPM has final responsibility for ensuring that all documentation necessary for clinical research regulatory compliance is maintained and updated as needed.
2. Clinical trial design concepts and parameters
A sound grasp of the clinical trials process is similarly a must for senior professionals in the team. Candidates for CRPM positions are thus expected to have a thorough understanding of key concepts and procedures relating to clinical research, including:
Phases 1 to 4 of clinical trials[5]
Investigational products (IPs) including INDs and IDEs
Design concepts in clinical trials - control, randomization and blinding
Subject screening and selection, retention and study compliance
Trial data input, quality assurance, analyses and reporting
Adverse Event (AE) documentation and reporting
3. Pre-trial preparation
In some ways, the most crucial phase of a clinical trial is the preliminary or preparatory phase, during which all groundwork must be laid for the smooth and efficient conduct of the actual study. This phase must typically achieve the following objectives:
Complete documentation for initiating clinical trials[6] – An IRB-approved clinical trial must have in place a host of documents covering important aspects of the study, including the investigator brochure (IB), trial master file (TMF), informed consent form, case report form (CRF), subject recruitment notice, laboratory protocol, study financial projections and so on.
Identification and preparation of study site(s) – Most clinical research studies are run across multiple sites, which may be hospitals, clinics or other health-care facilities. Identifying suitable sites, establishing collaborative agreements with site administrators, as well as setting up the required infrastructure to carry out trials at each site is crucial in preparing for a clinical research study.
Pre-trial publicity – The success of clinical trials depends on gathering large amounts of data from human subjects. Therefore, creating public awareness and interest right from the outset is critical to running clinical research studies effectively.
When setting up a study, the CRPM plays a central role in initiating and fulfilling each of the goals outlined above. Under the CRPM’s guidance, clinical research administrative staff including CRCs and CRAs work to put relevant documentation in place, prepare study sites and create public awareness about the study.
4. Active trial administration
The day-to-day running of an active clinical trial involves numerous administrative components, including:
hiring and training staff at clinical sites
tracking subject recruitment and retention
regulatory compliance in trial protocol, subject safety and data quality
Adverse Event (AE) documentation and reporting
oversight of trial drug storage and use
coordination among research teams across sites
tracking study finances
communication between study sponsors and clinical staff
electronic data capture (EDC) and management
timely renewal of necessary financial and regulatory approvals
These tasks are distributed among members of the investigative teams (PIs, co-PIs and other medical personnel) as well as administrative teams (CRAs, CRCs, QPPVs and other site staff). However, the CRPM is ultimately responsible for ensuring efficient administration that keeps the clinical trial on track.
5. Study termination procedures
Winding up a clinical trial similarly entails a number of administrative exercises. Here again, the CRPM must oversee execution, guiding team members in completing tasks on schedule and to the required standard:
site close-out (relieving staff, subject debriefing, equipment surrender)
final data consolidation and scrutiny
study completion documents for regulatory compliance
compilation of briefs and reports for regulatory authorities, study sponsors
The CRPM journey, Part 2: Expertise in project management[7]
Most aspirants to CRPM positions have usually been engaged in the clinical research process for at least a few years. Many have played administrative roles, working as CRCs, senior CRAs, QPPVs or site managers. In some cases, CRPM aspirants have clinical backgrounds, with expertise in drug research or medical monitoring.
Despite their hands-on experience with the clinical trials process, professionals wishing to transition to CRPM roles usually have limited exposure to the higher-level project management knowhow that is essential for an effective CRPM. This section provides a brief overview of the major project management domains relevant to clinical trial management:
1. Project and operations planning
A clinical trial is a major operation, involving a range of geographical and health-care settings, multi-disciplinary teams, as well as a complex array of regulatory and logistical requirements. Planning the stages, milestones and operations of a clinical trial is integral to assuring its success. Therefore, project and operations planning are the top priorities of a CRPM.
Some of the key elements involved in planning a clinical trial include:
Compiling a comprehensive study protocol describing the rationale, aims, design and methodology, data analysis plans, and trial procedure (/protocol)
Setting project goals, deliverables and milestones
Outlining a project personnel schematic that delineates team and sub-team structure and defines individual team member roles
Generating a responsibility blueprint with a clear break-up of tasks, targets and time-lines for each project goal and each team member
Creating a communication plan that outlines the method and schedule for relaying information, updates and requests between and within teams
2. Financial forecasting and tracking
One of the biggest challenges confronting a CRPM is keeping the trial within budget, while making sure that resources are optimally allocated to cover all necessary project-related expenses. Clinical trials often suffer from scope creep, that is, the inclusion of additional goals and deliverables to those originally outlined, resulting in the project overshooting budget and time-line.
It is therefore recommended practice for a CRPM to work with study sponsors and senior team members in compiling a budget management plan at the very outset of the project. Such a plan not only specifies financial outlay for each of the goals and deliverables of the clinical trial, it also provides for periodic budget revisions to account for factors such as expanded project scope, rising costs and supply-chain changes.
3. Human resource allocation and oversight
The section on project and operations planning highlighted the importance of creating a personnel schematic as well as a responsibility blueprint at the start of a clinical trial. Through this process, a CRPM is able to clearly define roles for various team members in meeting project goals and milestones, and their contributions to each project deliverable.
However, in order to ensure collaborative effort and a cordial work environment, it is essential for a CRPM to also formulate a transparent hiring policy as well as disciplinary policy. These may be specific to the clinical trial or apply broadly across the CRO, or Clinical (/Contract) Research Organization. Well-documented policies facilitate the CRPM’s role in sourcing and motivating the best-fitted staff to play different roles in the clinical team.
4. Clinical data management and quality control
The data from a clinical trial are often its most valuable project outcome. As such, effectively managing the input, storage, analysis and quality control of study data is one of the foremost responsibilities of a CRPM.
Before beginning the active phase of the clinical trial, a CRPM coordinates with team members to put in place the following aspects of data management:
physical data storage system (files, binders, shelves, cabinets, etc.)
electronic data capture (EDC) and management system
physical and electronic data security systems
data input procedure
data cleaning and quality monitoring procedure
data sharing (import, export, access across clinical sites and teams)
data analysis and visualization procedure
periodic data review and reporting procedure
5. Risk management and mitigation
Owing to the high stakes nature of clinical research, running a clinical research study carries numerous risks. One of the major responsibilities of a CRPM is to develop a risk management plan to identify and circumvent potentially risky situations, and to mitigate consequences to the project in cases where an unforeseen risk impacts the trials process.
Some sources of risk in the management of a clinical trial include:
failure to obtain or document subject informed consent
errors in following trial protocol (timing, dosage, subject screening, etc.)
data security breach leading to compromised subject confidentiality
inadequate insurance coverage for potential adverse events of study
financial constraints arising from changes in study funding
limitations on study scope due to revisions in regulatory policy
Bridging the gap: Using ACRPMC to transition from clinical research professional to project manager
The foregoing section outlined how, despite their strong base in clinical research, CRPM aspirants generally lack the advanced knowhow and managerial skills required for leading a clinical research project. CCRPS’ Advanced Clinical Research Project Management Certification (ACRPMC) provides an excellent tool for bridging this gap and bringing candidates up to speed in all domains of clinical research management.
The table below offers a summary of the focus areas of the ACRPMC, detailing how each contributes to enhancing a candidate’s expertise and eligibility for the role of a Clinical Research Project Manager.
ACRPMC Focus Area |
Contribution to CRPM Candidate Eligibility |
ADVANCED REVIEW - I: Regulatory compliance in clinical research |
i.Fundamentals of subject (/patient) safety, ICH-GCP E6, Sections 2 - 4 |
ii.Regulatory compliance – FDA-CFR Title 21, Forms FDA 1571, 1572, 3454, 3455 |
|
iii.Investigational New Drug (IND) & New Drug Application (NDA), Investigational Device Exemption (IDE) |
|
iv.IRB, Data Safety Monitoring Board (DSMB)[8] |
|
ADVANCED REVIEW - II: Clinical trial concepts |
i.Process & phases of clinical testing (pre-clinical and phases 1 to 4) |
ii.Importance of ‘control’ in testing[9], outcome validity, control methods (randomization, blinding) |
|
iii.Subject (/patient) selection[10]: screening & exemption criteria, selection & retention, study compliance |
|
iv.Clinical trial data management[11]: EDC, Clinical Trial Management Systems (CTMS), Interactive Response Technology (IRT), Randomization & Trial Supply Management (RTSM) |
|
v.Subject safety[12]: Risk assessment & Individual Case Safety Reports (ICSRs), Adverse Events (AEs), Adverse Drug Reactions (ADRs), Important Medical Events (IMEs) |
|
ADVANCED REVIEW - III: Pre-trial preparation |
i.Documentation[13]: Regulatory binder, Investigator Brochure (IB), Trial Master File (TMF), Case Report Form (CRF), Delegation of Authority Log (DOAL), etc. |
ii.Site preparation[14]: Site Selection Visits (SSVs), Site Qualification Visits (SQVs), site management agreements, investigator selection |
|
iii.Pre-trial publicity: Advertisement for subject recruitment & retention |
|
ADVANCED REVIEW - IV: Active trial management & trial termination |
i.Clinical trial protocol compliance, deviations & violations |
i.Clinical site monitoring: subject safety, drug storage & safety, regulatory compliance |
|
ii.Data monitoring: data security & access, Quality Management System (QMS), Quality Control (QC), Key Quality Indicators (KQI) |
|
iii.Team monitoring: communication & collaboration, adherence to trial & safety protocols, research integrity & fraud |
|
iv.Reviews & audits[15]: document & site audits, data audits, financial audits |
|
v.Study termination: site close-out visits, data consolidation, subject debriefing |
|
EXPERTISE BUILDING - I: Clinical project management fundamentals |
i.Project management basics[16]: key concepts, Project Management Body of Knowledge (PMBOK) |
ii.Skills of a project manager: technical, administrative, human resources, public relations |
|
iii.Clinical project stakeholders: sponsors, IRB, investigative & administrative staff, site administrators |
|
EXPERTISE BUILDING - II: Advanced clinical project management |
i.Project planning & tracking, progress evaluation metrics |
ii.Financial management[17]: project budgeting, site budgeting, advertising budget, payment tracking and management |
|
iii.Risk management & problem solving[18]: problem solving managerial skills, identifying risks, avoiding failures, resolution of project problems (protocol deviations, compliance failures, project delays) |
Fast-track to CRPM with the ACRPMC advantage
From the above summary table, it can be seen that the ACRPMC[19] program of CCRPS covers the entire spectrum of competencies required for managerial clinical research professionals. In addition to providing in-depth training in clinical project management concepts, tools and techniques, the program includes a review of foundational as well as advanced knowledge of the clinical trials process.
Aside from its comprehensive curriculum, certification through ACRPMC offers major benefits over conventional clinical research management courses, both online programs as well as those offered by colleges and universities. Some of the outstanding advantages of ACRPMC are:
1. Foundational review plus advanced training
The ACRPMC program stands apart from other online programs in its unique ability to launch as well as advance careers in clinical research. The program is equally effective at helping an aspiring graduate (BA in science or BS degree) to gain a competitive advantage in the clinical research job market, as it is at catapulting a senior clinical researcher into a managerial position.
For CRPM aspirants in particular, the ACRPMC provides an unrivaled opportunity to brush up on the basics, in addition to acquiring valuable knowhow in clinical research project management, before embarking on the applications and interview process.
2. Over 100 training modules (250 hours of coursework)
The course comprises over 100 modules. The advanced review focus areas address all aspects of the clinical research process, including important investigative concepts (trial design, randomization, subject screening, data analysis, etc.) as well as administrative processes (maintenance and updating of regulatory documents, case reports, trial logs, etc.). Additionally, the expertise building focus areas of the ACRPMC cover basic and advanced project management concepts and skills that equip candidates to tackle the challenges of leading a clinical research study.
3. ICH-GCP E6R2 compliant
Designed by experienced Clinical Research Project Managers, the ACRPMC curriculum is fully compliant with the regulatory requirements of the ICH-GCP E6R2. The course coordinators additionally ensure that the content is regularly updated to reflect revisions to policy and new requirements.
4. Immediate enrollment and fast-tracked certification
Unlike college and university courses, trainees can enroll in the ACRPMC with just a few clicks. In addition, the self-paced course design allows students to skim through familiar topics, while giving more time and attention to new material. This is especially advantageous for working professionals and senior clinical research professionals wishing to refresh their knowledge. Trainees able to dedicate several hours per day to the program can complete the ACRPMC in as little as two weeks.
5. Multiple accreditations and industry-wide recognition
For ACRPMC-certified trainees, the solid credentials of the program confer a huge benefit when applying and interviewing for clinical research positions. Like other CCRPS courses, the ACRPMC is also accredited to ACCRE (Accreditation Council For Clinical Research and Education), ACCME (Accreditation Council for Continuing Medical Education) as well as ACPE (Accreditation Council for Pharmacy Education).
6. Clinical research résumé building and interview preparation
The final modules of the ACRPMC help trainees in building their ability and confidence to face interviews for clinical research positions. Trainees completing the comprehensive final examination not only have immediate access to a digital certificate, but are eligible to receive a letter of recommendation (LoR) that can strengthen their clinical research job application.
As the clinical research industry continues to grow at an unprecedented pace, there is rising demand for Clinical Research Project Managers who can blend their skill and experience in clinical research with savvy, expert project management abilities. As a professional clinical researcher, the ACRPMC program offers you an unmatched opportunity to refresh existing knowhow and acquire new competencies, thereby leveraging your rise to the top echelons of management.
If you are a fresh graduate, the ACRPMC represents an unequaled, early-career edge in the clinical research arena. By equipping you with foundational as well as advanced knowledge and skills, ACRPMC certification does not merely improve your chances of securing an attractive clinical research position, it assures the rapid upward growth of your career graph.
Advanced Clinical Research Project Manager Certification (ACRPMC) program advantages.
References
https://www.indeed.com/jobs?q=Clinical%20Trial%20Manager&l&vjk=311beece3c2d98b6
https://www.payscale.com/research/US/Job=Clinical_Trial_Manager/Salary
Dixon JR. 1999. The international conference on harmonization good clinical practice guideline. Quality Assurance. 6(2): 65-74. DOI: 10.1080/105294199277860
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=312.32
https://ichgcp.net/8-essential-documents-for-the-conduct-of-a-clinical-trial
Cullen H. 2016. Effective project management for clinical trials: A business approach. Ebook: https://www.imperialcrs.com/files/Project_Management_Ebook_Final.pdf
Jüni P, Altman DG, Egger M. 2001. Assessing the quality of controlled clinical trials. BMJ. 323(42). DOI:10.1136/bmj.323.7303.42
Farrell B, Kenyon, S, Shakur H. 2010. Managing clinical trials. Trials 11(78) DOI:10.1186/1745-6215-11-78
von Itzstein MS, Hullings M, Mayo H, Beg MS, Williams EL, Gerber DE. 2021. Application of Information Technology to Clinical Trial Evaluation and Enrollment: A Review. JAMA Oncol. 7(10):1559–1566. DOI:10.1001/jamaoncol.2021.1165
Yao B, Zhu L, Jiang Q, Xia HA. 2013. Safety Monitoring in Clinical Trials. Pharmaceutics. 5(1):94-106. DOI: 10.3390/pharmaceutics5010094
https://files.nccih.nih.gov/s3fs-public/CR-Toolbox/Regulatory_Binder_Checklist_ver3_07-17-2015.docx
https://www.appliedclinicaltrialsonline.com/view/selecting-study-appropriate-clinical-sites-3-steps
https://www.clinicaltrialsarena.com/news/how-to-prepare-for-an-fda-site-inspection-5739632-2/
PMBOK® Guide (7th ed.). 2022. Project Management Institute.
Appleson M. 2015. The importance of budgeting in clinical trials and how a budget can prevent billing errors. Clin Invest. 5(5): 437-439. DOI: 0.4155/CLI.15.11
Robinson M. 2005. Clinical trials risk management (1st ed.). CRC Press. DOI: 10.1201/9781420037654
https://app.ccrps.org/courses/Advanced-Clinical-Research-Project-Manager-Certification
Clinical Research vs Clinical Trial
Many people use both terms interchangeably in their clinical research discussions. Although we would not say that it’s a totally wrong thing to say or do, but it is important for us that you know the difference between both terminologies.
Clinical research is the study of people’s health and the illnesses in people. The purpose of clinical research is to prevent diagnose and treat illnesses but more importantly as well, to prevent them. Clinical research describes many elements of scientific investigation. It helps to transform basic medical research that are carried out in the laboratory into new treatments and useful information that are beneficial to the patients. It usually involves the participation of humans.
On the other hand, a clinical trial is an experiment that is designed to create answers and clarifications about possible new treatments or new ways of administering a previously known treatment for more effectiveness. Clinical trials are carried out to determine the safety and effectiveness of using new drugs or types of treatment. It's a long and meticulous process that takes years to finish.
Clinical trials are aimed at evaluating medical, behavioral, or surgical intervention of drugs or treatments in people. They study the role of caregivers, develop tests to find a disease early before there are visible symptoms, and look at how to make life a little better for people living with chronic health problems or life-threatening diseases. Clinical trials cannot be approved by the U.S. Food and Drug Administration (FDA) until clinical research professionals have performed laboratory tests and studies in animals, and it has been proven safe and efficient.
The main distinction between clinical research and clinical trial is that clinical trial is a part of a clinical research.
Clinical trials are part of a long and careful process that is carried out over a long period of time. Firstly, the new treatments are studied in the lab by doctors. Then they usually test the treatment on animals like apes, rats etc, depending in if the fit particular criteria.
If the treatment shows good promises in the animals, then they go ahead to test it on human subjects through clinical trials in the clinical research labs.
So we can safely say that clinical trials is one of the many phases of clinical research.
If you are a prospective clinical researcher, that is, if you are interested in a clinical research career, you can’t afford to miss out on little but vital details like this. Details and knowledge that come from experience are available for you at ccrps.org, where you will work with people willing and ready to help you launch your career forward.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
How to Get into Clinical Research
If you want to get into the clinical research, there are a few steps you will have to go through. In this article, we'll be taking you through the steps and some common questions.
What does Clinical Research entail?
Clinical research entails testing medicines or products for safety and effectiveness. It involves working with patients during extended experiments to record and quantify the effect that different medicines produce. It is a highly regulated field due to the use of human subjects.
Salary earned ranges from $39,000 to $87,000. However, the more one acquires certifications and qualifications, the more opportunities you have. That means you can choose the best position and salary for you.
The first step is to get an education.
Earning a bachelor's degree in a life science or health related discipline by taking specific courses like medicine, pharmacology, biology, molecular biology, genetics, anatomy, biotechnology, nursing, physiology, chemistry, or bioengineering will equip you with the necessary and relevant medical knowledge, science, and technicalities to qualify you into practicing as a clinical research.
Taking courses that are relevant to the clinical research that will give you the necessary experience and knowledge that are relevant to clinical research conducts. Applying for the courses offered at your university or from professional organizations like Certified Clinical Research Professionals (CCRPS) and Association of Clinical Research Professionals (ACRP) are a great place to start. These courses will include topics such as study designs, Good Clinical Practices (GCP), research ethics, drug development cycle, regulatory affairs and U.S as well as international requirements.
You can get a certification from a reputable organization, such as CCRPS, ACRPS, and Society of Clinical Research Associates (SOCRA), as long as you have a Bachelor's design and at least one year of experience in clinical research. This certification allows you gain more access into the clinical research industry.
When you are taking your courses, make sure you study the ICH GCP guidelines and ethics thoroughly. Training in the International Conference on Harmonisation (ICH) Good Clinical Practices (GCP) ethics and guidelines improves your chances of getting hired greatly.
Remember to keep proper documented records of your certifications as well as your education. This will save you a lot of time and stress.
The next step is to gather experience.
The following are ways you can gather experience;
Volunteer - Look for volunteering opportunities around your area and volunteer to help with the projects that will be carried out in the clinical research industry. This helps you get closer to the professionals as well as what to expect at the job. You might be chanced to start out as a clerical worker or a data entry staff, but not to worry, you'll be able to work your way up the ladder. Once you are in the field, you can discuss the possibility of applying for a position with the place you are working at in the future. Employers will be more likely to consider you when there is someone in the company vouching for you.
You can volunteer at clinical research professionals organizations related to the clinical research field or medical field, medical centers or hospitals, International Review Boards (IRB) or Research Ethics Committees.
Research Projects - Most entry level jobs require around two years of experience. Taking up clinical research monitoring projects for a few years can really help you get the experience you need. You can also conduct research studies with human subjects during your pursuit of a bachelor's degree or graduate degree.
Internships - Seek out an internship with medical firms, biotechnology, and pharmaceutical companies while you're still in college. You may or may not get paid as an intern but it's nothing compared to the experience you'll gain that will be needed for your venture into clinical research.
Finally, the last step is to apply for entry-level positions.
This is the last step that will get you right into the world of clinical research. After all your education and gathered experience, you cam apply for an entry-level position as a Clinical Research Coordinator (CRC) or a Clinical Trial Assistant (CTA). Both positions only require around two years of experience and they are the ones that you can qualify for. Applying for high-level positions you don’t qualify for yet will only waste your time.
Generally, you should apply for positions at smaller firms. It's okay to aim for positions at the biggest pharmaceutical companies and clinical research organisations (CROs), but as a newcomer, the competition may just be too high. So, why not just apply for positions at smaller firms and work your way to the top?
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Joining the ICH-GCP Clinical Research Program
Clinical research is one of the best career path to get involved in. They have positions for every lifestyle and personality. However, a solid understanding of the rules and regulation of the field is critical for every professional in the field. In this article, we will talk about the GCP and how it should impact the way you approach clinical research.
About the GCP
Good Clinical Practice (GCP) is an international standard provided by the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH). GCP enforces strict guidelines on ethical aspects of the clinical study.
Individuals who are aware of the significance of very good clinical practice guidelines and the role of the clinical research know the importance of online GCP programs.
Medical professionals who have successfully completed their clinical research training program can improve their expertise on regulatory authorities and approval specifications for the marketing of the healthcare products. This can make a big change in their work life. A good training program can help them improve their proficiency in the best use of medical data, databases and data evaluation. This will help them build proficiency in the communication, research and management, educational approaches, problem-solving and other things related to the clinical research.
Make a better informed decision
The salary is one of the main factors considered by people planing to choose a degree or certification program. Here, you can find the average clinical research salary in your area and decide on whether a career in the clinical research.
If you wish to become a certified clinical research professional, then you have to find out and join in the program specially designed for career.
Clinical research involves drug development as well as healthcare research. Experts in this profession engage in the patient-focused research and make use of every chance to be successful in the clinical research career.
CCRPS visitors learn about clinical research and are able to make an informed decision to join the best program for them. When you have questions, you can have instant assistance from the experienced and committed professionals. We ensure our students understand about everything associated with the clinical research degree program and use every opportunity to be successful in the clinical research sector.
Improve your resume now with online clinical research certification or membership in clinical research societies.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
What Is a Clinical Research Coordinator
The clinical research is a ground breaking field in the world of medical advancement and as such, has a wide variety of professionals that are changing and channeling the drive.
We will be taking a look at one of the highest paying certified clinical research professionals, clinical research coordinator.
CLINICAL RESEARCH COORDINATOR
The clinical research coordinator is responsible for conducting trials as per the GCP guidelines under the supervision of the Principal Investigator (PI). Although the PI carries the overall responsibility for performing the trial, the CRC is the heart and soul of the clinical trial and that, ultimately, it is the CRC who carries ahead the research objectives and ensures the success of the clinical trial.
Having a certification in clinical research in other to become a clinical research coordinator is not compulsory, but certification enables one to show that they have met the necessary requirements and have gained job-relevant knowledge and skills. This distinction is very important to pharmaceutical companies and contract research organizations, who frequently hire clinical research staff.
EDUCATIONAL REQUIREMENTS
• High school diploma and 6,000 hours of experience.
• An associate degree in clinical research fields amd 4,500 hours of experience, or
• A Bachelor's, Master's, or Registered Nurse degree and 3,000 hours of clinical research experience.
You can come from a variety of medical sciences or health related fields, or from a nursing background as a RN. Courses offered in hospital and clinical related ethics, team management, and research methodologies will be especially valuable.
Who Will Hire Clinical Research Coordinators?
Pharmaceutical Organizations
Contract Research Organizations
Universities
Hospitals
SPECIALIZATIONS
They focus on the following;
1) Participating in preparation and management of research budgets and monetary disbursements.
2) Informing patients or caregivers about study aspects and outcomes to be expected.
3) Communicating with laboratories or investigators regarding laboratory findings.
4) Ordering drugs or devices necessary for study completion.
5) Directing the requisition, collection, labeling, storage, or shipment of specimens.
6) Arranging for research study sites and determine staff or equipment availability.
7) Reviewing scientific literature, participate in continuing education activities, or attend conferences and seminars to maintain current knowledge of clinical studies affairs and issues.
SALARY
Clinical research coordinators have had a positive trend of pay for experience. Therefore a CRC with less than 5 years experience is paid an average total compensation of $43,000 (based on 1,237 salaries). Employees with 5 to 10 years experience can expect the average compensation of $51,000 (based on 429 salaries). Employees with 10 to 20 years of experience is paid an average compensation of $55,000 (based on 265 salaries). Finally, employees with more than 20 years of experience can expect a compensation of $62,000 (based on 52 salaries).
While many stay CRCs their entire career, most will move to higher postions after 3-5 years of experience. One of the most popular careers CRCs switch to is CRA, which can pay up to $300k.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
How to Become a CRA and Succeed
The Clinical Research Associate (CRA) is one of the most popular entry-level job in the clinical research sector. It is also one of the most competitive and lucrative positions for new hires. How do you stand out from hundreds of other applicants? You need to build field intelligence and experience.
Knowledge is power in the clinical research industry. For example, did you know that there are loads of differences between the clinical trial and clinical research? If you pay attention to learning the critical details of the field, employers will see and appreciate the effort you put into understanding the field.
There are many institutions that provide clinical research courses for aspiring professionals. However, CCRPS' reputation for the best-in-class programs is offered at the competitive prices is unbeatable. Our world-class facilities is known for teaching both basic and advanced lessons that help professionals in clinical research.
Improve your Clinical research job qualifications
To become a CRA, you’ll need an undergraduate degree in medical science, life science, biotech or nursing and a certification. Alternatively, you can use your diploma from a clinical research program from a reputable institution. If you have a graduate degree in the clinical research, then you are eligible for senior level positions and a higher pay grade.
In addition to your degree, individual should demonstrate their interest by joining a clinical research associate program from a trustworthy institution. There are many specific skills required to become a qualified clinical research associate, and they aren’t always taught in every programs. You must understand the healthcare system, clinical research, healthcare regulation and procedures and other things for successfully regulating the growth of the healthcare products.
Presently, individuals who want to become a CRA should be capable of preparing a clinical development plan and ensuring the clinical trial data. They should have the overall understanding of the liabilities and responsibilities of performing the study with human subjects. They have to know all the challenges and restrictions of implementing and retaining databases.
At CCRPS, our program will walk you through what you need to know to become a CRA. Our AACRE accredited program is curated by real life professionals with years of direct experience in clinical research. Best of all, you can learn at your own pace. With our flexible online learning model, you can take your education anywhere you do. Enroll and start working to your dream career through our online CRA certification program today. If you’d like to learn more, check out some of our other CRA articles below.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
What is a Clinical Research Epidemiologist
Few careers can impact human health and global policies the way a clinical research epidemiologist does. People walking this careers in public health path have the exciting task of discovering how and why diseases happen and prevent the spread and recurrence of these diseases.
A clinical epidemiologist studies diseases and provides information to public health networks about disease prevention and control.
Requirements for Epidemiologists
EDUCATION
Education in the following fields is highly useful in preparing for a future career as a clinical research epidemiologist:
• Biological sciences
• Statistics
• Physical sciences
• Immunology
• Biostatistics
SKILLS
A master's degree with a specialization in public health is the common education requirement for clinical epidemiologists.
Epidemiologists are often called upon to provide community outreach and public health information services, which makes skills such as critical thinking, communication, and a knack for teaching important for success in the field.
SPECIALIZATIONS
There are several specializations people interested in clinical research epidemiology can pursue. These lead to different, but uniquely rewarding career paths. The following specializations are among the most common:
• Infectious diseases
• Chronic diseases
• Bioterrorism
• Injuries
The key is for students to find specializations that spark their interests.
SALARY
Pay for an epidemiologist is $65,270 per year or $31.38 per hour. The income varies greatly depending on the industry. These professionals work in a variety of environments, such as an office, lab, or a medical setting with doctors. Check here for postings near you.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Job Training for High School Graduates Using a Clinical Research Institute for Online Clinical Research Certification (CTA, CRA, CRC)
Choosing the right job course online is a challenging task for almost everyone new to certification programs. There are so many different attention-grabbing packages of affordable clinical research programs, how do you know which one is for you? People who focus on the successful institution specializing in the clinical research programs are most likely to find a program they can truly learn and benefit from. Visit CCRPS, a major AACRE accredited U.S Organization, and find courses provided by the certified clinical research professionals. You will get guidance about the programs in the clinical research that goes beyond your expectations. Their education will help you make a better-informed decision to choose and join in one of the best programs without complexity in any aspect.
Job training for high school graduates
Future professionals with an ambition to choose and join the best courses should consider important things like the overall value of the course and how they contribute to career development. At CCRPS, we not only offer a description of what to expect in the field, we also offer real life suggestions from experts already working in clinical research.
Once you have joined our program, you can learn more than a few important things and enhance your career profile. You will get different career options and make positive changes in your career life. You will be satisfied with the successful method to prefer and join in the suitable job after a comprehensive analysis of an array of important things.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Clinical Research Certification Can Lead to a Higher Salary
If you like science and you want to work for an exciting, in-demand field, then clinical research may be a great fit for you. The clinical research field is developing at an amazing rate and opening up a broad scope of job opportunities for qualified professionals.
There is a huge demand for professionals that specialize in clinical data management, test management and managing ethical problems related to clinical research. This presents an impressive career choice with extensive growth potential.
With such a huge expansion of clinical test labs, clinical research degree programs is growing rapidly.
Your clinical research degree from an accredited society:
Clinical research courses are designed to change those with a Life Sciences Bachelor’s into skilled professionals that will bring value to the firms that hire them. Classes include clinical research basics and theories, internships, and improvement of soft skills that will improve your employment prospects.
Getting a Master’s in Clinical research degree will qualify you for a broad range of financially satisfying career paths. The MS in Clinical Research can pave way for those with a bachelor degree, as well as clinical scholars, physician-scientists, and biomedical researchers.
This degree trains you to manage patient-oriented study, directly communicating with human problems to completely know the disease, the growth of healing interventions, and the execution of clinical tests. You will discover how to handle epidemiological and behavioral investigations as well as explain problems associated with outcomes-based research. In addition, you will learn to strengthen your grant reporting and information analytic abilities.
If you are considering a clinical research degree, there are some fields and employers you should consider:
Contract Research Organizations (CRO)
Biotech Companies
Pharma Industries
Pharmacovigilance
Medical Writing
Patient Recruitment Organizations (PRO)
Regulatory Affairs
Clinical Data Management
Research labs
Logistics Services
Options for research basics
Clinical Trial Audits
Every clinical research quotes require a chief researcher to lead the study. These researchers could be any of the following:
Researchers
Professor
Medical Practitioners
Specialists
In addition, the project needs a team of:
Clinical Research Associate (CRA) (click for certification course online)
Clinical Research Coordinator (CRC) (click for certification course online)
Biostatisticians
Research site manager
Research Auditors
Quality Assurance specialists
Marketing Executives
Regulatory Affairs Manager
Translators
Lab staff
In clinical research, there is a place for every kind of talent. Check here to see the positions open near you. For more details and training you can check ccrps.org.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Why Do People Choose Online Clinical Training Courses?
People wish to have the perfect platform to perfect their clinical research. As the clinical training program courses become more popular among people, in some cases there are people who simply choose clinical research training courses without knowing its roles and responsibilities.
When people decide to pursue clinical research course, there are several available ways, such as training programs from clinical research institutes or in online training programs.
Although there are many clinical research institutes, most prefer taking clinical research training programs to make their career stronger in the medical field. This is mainly because when people choose courses in clinical research institution or any research centers, it would costs $30,000 to $ 50,000 while online courses cost much less.
Facilities provided in clinical research training in institutes:
You must be wondering how an online course might differ from a physical course. Online training courses are very similar to the courses offered in clinical research institutes. People can choose their desired specialization and attend daily sessions. Once they have completed all sessions and passed the course examination, they receive a course completion certificate.
Although the online clinical courses are similar to the courses offered in clinical research institutes, there are some facilities which are offered only in the clinical research institutes and research centers:
In clinical training institute, students can experience clinical research operations with patients.
Students are allowed to do test on various drugs and medicine, analysis them in traits test center.
Based on the analysis test report, student can learn to decide whether the drugs are safe for market use or need to be sent for further analysis.
These facilities are only provided to people that choose clinical research courses from clinical research centers and institutes. These skills and experiences might be important to potential employers, so be sure to check the requirements on all job postings. For more details regarding this you can check on ccrps.org or visit us to learn more about our online clinical research certification programs.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
What Made Clinical Research Courses Popular in the Medical Field?
when students wish to have strong career in the interesting medical field, they often become clinical researchers. The clinical researcher career is a popular choice because it brings several career as well as financial benefits. However, when you decide to choose clinical researcher as a career, then you should be aware of the roles and responsibilities involved in them.
Since there are so many available clinical research training programs, it is important that people know what roles and what specialization they want to go into. For example, when people complete their course or training programs from licensed centers, they could get a clinical research organization jobs in pharmaceutical companies, medical research organizations, and in clinics. Although there are many courses available, people who knew going into training knowing exactly where they wanted to work would benefit more from their classes.
Due to the popularity of the field, those who get their Master’s in Clinical Research have an advantage. Their degree could get them better positions and pay than their counterparts.
How to choose the best clinical training institute for master’s course online?
Many people show an interest in completing their Master’s in Clinical Research, but in real life getting a Master’s in Clinical Research is not easy. In order to complete the course, people would need $30,000 to $50,000. Course from the top research centers would cost even more.
To tackle this situation, most people look to get their Master’s in Clinical Research online. Many wonder if online courses would be on par to trial centers training. The answer is that when students decide to do their Master’s in Clinical Research online, they find that the courses are very similar to classroom training.
Moreover, by taking online courses people can avoid several additional costs like travel, high tuition, and taking time off work. The only thing that different is that in an online master’s course, people can’t experience hand on training with patients, drugs and medicines.
When deciding to pursue an online masters, below are some other information you should consider:
There are many training centers that offer online Master’s in Clinical Research with low tuition fees. So people need to compare and choose the best among them.
Check whether the training center provides clinical research quality assurance training across all specialization. This speaks to the quality and comprehensiveness of the courses offered.
While comparing centers based on their courses, check whether the center would offer help with job placements too.
Before committing to anything, check jobs near you to see if an online course completion certificate is valid for the posting.
Thus based on all above factors, you can confidently choose the best clinical research training institute to pursue your Master’s in Clinical Research.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
A Career as a Clinical Researcher and Its Benefits
Clinical research is a multidisciplinary, multinational and multi billion industry. Now days the pharmaceutical industries, one of the top employers for clinical research work, are becoming a fastest growing sector that boosts the economy of the clinical research to the rapid development. As compared to the previous years the clinical research field has undergone a remarkable evolution in the power, sophistication and scope on its methodologies. There is a remarkable change in the approach to the data analysis, data collection and experimental design where these are found to be the basis for the strong clinical research career. It is the next big thing that will create a wider scope of job opportunities in the field of life science.
Numerous advances topics and opportunities are cited in the clinical research graduate programs. This helps individuals learn about new developments in the field. The following are the some of the benefits of completing the graduate program in the field of clinical research. They are:
When you complete the graduate program in the clinical research, you qualify for positions that have significantly better non financial and monetary benefits than other areas of the industry.
You will typically enjoy a high base salary and expect an annual bonus from the company. Depending on your position, the company will typically be providing the vehicle facility for personal usage too.
You will be also getting the food allowances and health insurance profits. The company will also be providing you the gadgets like a mobile phone, laptop and all in one printer for working from home/remote working.
When you have the knowledge and skills from the clinical research education program, you have the opportunity to work with other high skilled staffs as well as the perfect work and life balance.
How to obtain a career in the field of clinical research
Clinical research is an expanding area of the work that is gradually generating the more interest for many people. Fortunately, there are countless opportunities in clinical research for the doctor of nursing practice and anyone can pursue a career in the field of the clinical research.
One of the most important benefit of pursuing a clinical research program is the wide range of jobs vacancies that are available in this field.
Some of the typical tasks in clinical research is taking part in the development planning, project management, management and site selection, feasibility studies, development planning, and project management. In order to get into this clinical research profession it is important to have the degree in any of the following subjects like medicine, nursing, biology, immunology, dentistry, chemistry, and pharmacology. These fields are where most of the clinical roles and jobs are available. You can check here to check out positions available near you. For more details and training you can check ccrps.org.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Everything You Should Know About CTRI UCSD
The UC San Diego Altman Clinical and Translational Research Institute, better known as CTRI UCSD, acts as the hub for translational medicine. It helps in delivering the scientific breakthrough patient care through clinical trials.
They offer participants early access the treatments, which would one day re-define the standards of care. Every year, the clinical research volunteer program would enroll the participant with the hundreds of the clinical trials for promising the investigational drugs, devices and the treatments.
If you want to become part of a research institute like CTRI UCSD, clinical research education can help you.
Become well versed in the field that you really like.
Pave a way for you to shine and know about the field.
Helps you to do more research work based on the type of the field that you have chosen.
Things that you want to know about clinical research
The clinical research acts as the branch of the healthcare science. If you are interested in the field, doing the clinical research online training program that is associated up with clinical research associates (CRA), clinincal research coordinators (CRC), or clinical trial assistants (CTA) could be a great way to get started.
Different research training programs could vary based on the student locations, time and the learning demands.However, CCRPS’ self paced online modules is reliable and contain interactive tutoring sessions that promotes monitored competency. Classes provide the foundation that will help you break into the field.
The ccrps.org paves a way for you to get trained up in the clinical research. Before choosing it, you can go through all the reviews that are available to give you a better idea of our courses. To know more details about it you can gather the information for training in clinical research here.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
2019 Clinical Research Salaries
Want to know how much a clinical research job post salary. Read our blog post for clinic research job post salary. Here you can get the info about the post description and salary numbers.
The average salary for clinical research professionals is $63,000, but there are lots of different clinical research professionals in the clinical research industry and we will be taking them one by one to see their differences in salaries.
Clinical Research Associate (CRA)
CRAs earn $63,030 annually on average. Most join as junior clinical research associates (CRAs). They learn the life cycle of a clinical trial, how to monitor the clinical trial activities at different stages, and everything about clinical data monitoring and management.
Clinical Research Coordinator (CRC)
CRCs earn $48,739 annually on average. The clinical research coordinator is responsible for conducting trials and carrying our research objectives. Most significantly, CRCs are usually engaged in vital duties for the PI, like taking care of the informed consent process and making sure that the site staff is in compliance with the protocol. The CRC’s main responsibility, as with all clinical research staff, is to ensure the protection and well-being of the patients participating in the study.
Senior Clinical Research Associate (CRA)
SCRAs earn $97,813 annually on average. The senior CRAs deal with more complex clinical trials and site management issues than junior CRAs. They are there to highlight the importance of international research as well as the role of the ICH GCP process. They also give updates on the overview of current legislative requirements, including guidance on substantial trial modifications, improving the recruitment process of subjects and site staff, as well as how to prioritize and upgrade clinical trial monitoring tasks and activities, accurate monitoring and reporting.
Biostatistician
Biostatisticians earn $70,732 annually on average. They are involved in every step of clinical research, including trial design, protocol development, data management, and monitoring, data analysis and clinical trial reporting. They are also involved in data management protocol development and design study implementation data analysis and reporting and study monitoring.
Clinical Project Manager
CPMs earn $84,048 annually on average. The job of a CPM includes planning and managing all aspects of a clinical trial. In order to conduct a clinical trial adequately, CPMs manage a team of CRAs and Clinical Specialists and act as a link between the study sponsor and the clinical trial site.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course