Clinical Research Associate Salary - What's the pay for a clinic research associate?
Here's What You Need to Know to Get a Clinical Research Associate Job
What's the pay for a Clinical Research Associate?: $61-$110K
What's the pay for a Clinical Research Associate?: $61-$110K
A Clinical Research Associate (or Monitor) is hired either in-house (“the trial site”) or externally (by the sponsor or CRO) to review clinical trial data and ensure that investigational therapies are tested ethically and scientifically through performing site visits that review files like patient medical notes in order to ensure quality of trial data. The catch 22 of clinical research associate jobs is that the ICH GCP guidelines require both education AND experience in order to work in this role, so getting your foot in the door is tough. Once you get experience, your education (i.e., certifications or degrees showing understanding of additional responsibilities) can help promotes you quickly through the CRA career ladder. For those looking to understand more about these guidelines, the ICH-GCP course might be a crucial step.
How to become or get promoted as a clinical research associate?
Having a certification through CCRPS’s accredited Advanced Clinical Research Associate Certification (CRA) course can help professionals 1) get promoted 2) get a raise 3) improve efficiency 4) get hired as a Clinical Research Associate. Additionally, those interested in broader roles in clinical research may consider the Clinical Research Coordinator course, the Pharmacovigilance Certification, or the Clinical Trials Assistant Training.
How much does a Clinical Research Associate make in the United States?
The average Clinical Research Associate salary in the United States is $61-110K. Salary ranges can vary widely depending on many important factors, including education, certifications, additional skills, and the number of years you have spent in your profession. For those looking at a leadership role, the Advanced Clinical Research Project Manager Certification or the Advanced Principal Investigator Physician Certification could be relevant. Furthermore, the Medical Monitor Certification can enhance skills for those specifically interested in medical monitoring aspects.
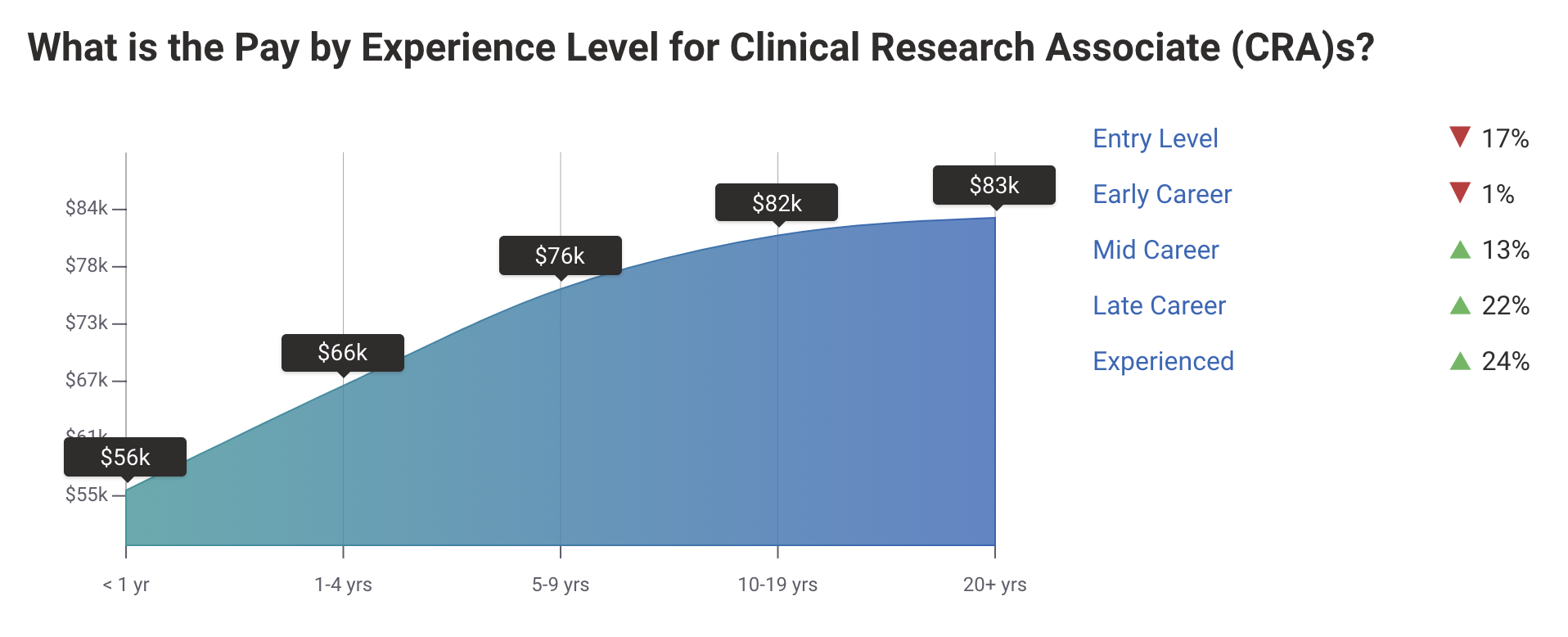
Clinical Research Associate (CRA) Salary
Per Payscale “An entry-level Clinical Research Associate (CRA) with less than 1 year experience can expect to earn an average total compensation (includes tips, bonus, and overtime pay) of $55,588 based on 203 salaries. An early career Clinical Research Associate (CRA) with 1-4 years of experience earns an average total compensation of $66,245 based on 1,118 salaries. A mid-career Clinical Research Associate (CRA) with 5-9 years of experience earns an average total compensation of $76,086 based on 294 salaries. An experienced Clinical Research Associate (CRA) with 10-19 years of experience earns an average total compensation of $81,540 based on 157 salaries. In their late career (20 years and higher), employees earn an average total compensation of $83,342.”
Determine CRA Salary by location using Payscale
Salary: Clinical Research Associate
Research Associate Salary resource: Click here to see the CRA Salary Range from 1,800+ employers
Clinical Research Associates Pay: Clinical Research Associate Salary in United States (by city)
Durham, NC - $97K
New York, NY, Irvine, CA, Houston, TX - $95K
Philadelphia, PA - $92K
Atlanta, GA - $84K
Raleigh, NC - $82K
Chicago, IL - $79K
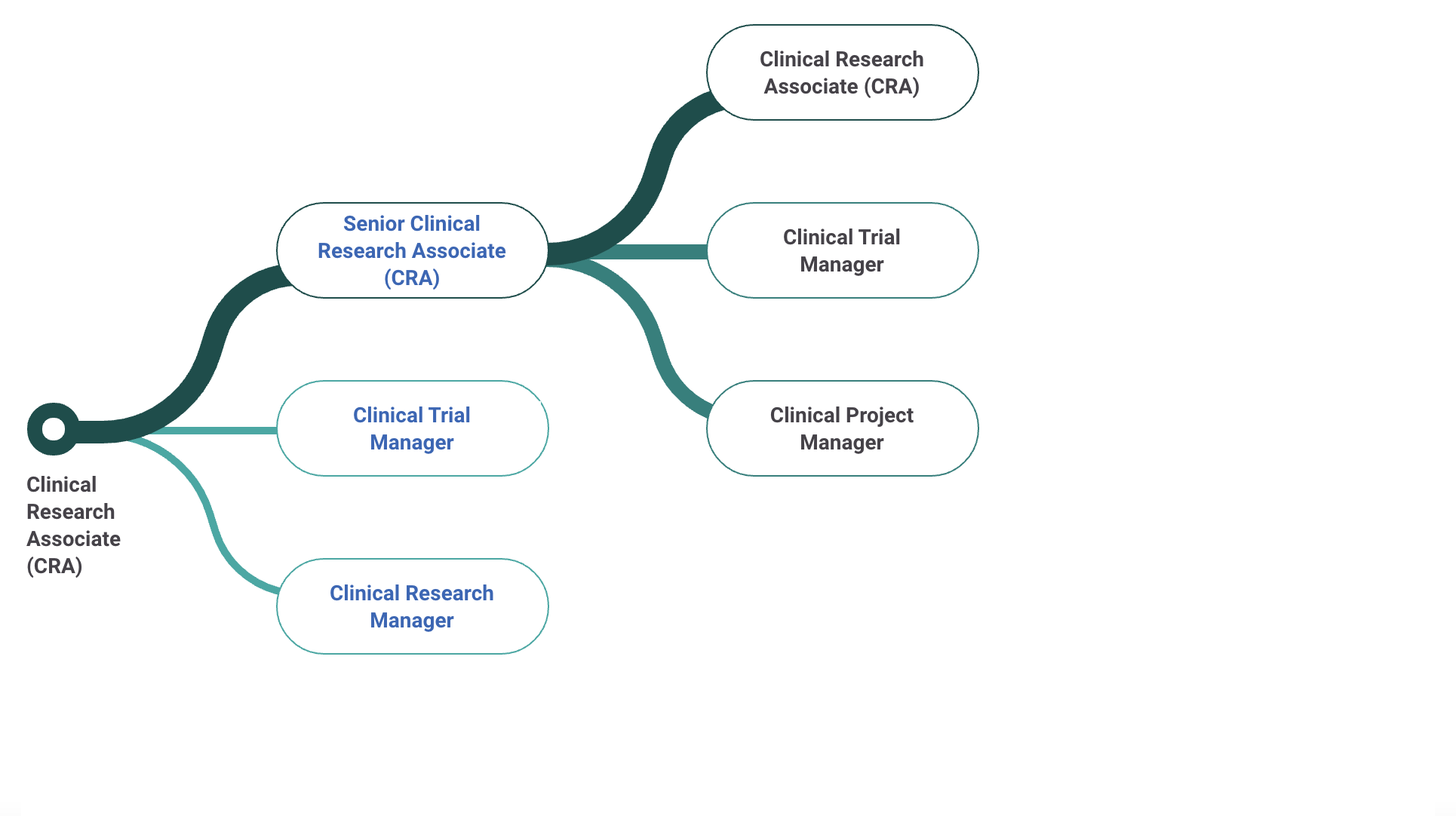
Senior Clinical Research Associate Salary
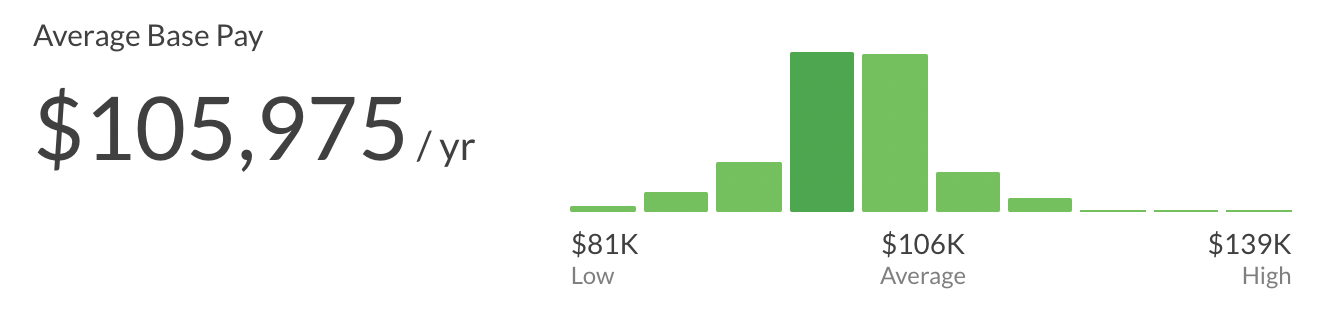
The average salary of a Senior Senior Clinical Research Associate is ~105K per year ($54/hour, $2k/week, $9k/month). This can range from $81k to $139k.
Starting Salary of a Clinical Research Associate Position is between $60,000 and $65,000
Some employers may prefer hiring entry-level clinical research associates with less experience in clinical research so they can learn their job functions by training under Senior CRAs or CRA IIs.
Clinical Research Associate II Salary is $86,677 / yr
CRA II @ PPD: $84,733/yr
CRA II @ PRA Health Sciences: $96,017/yr
CRA II @ IQVIA: $79,412/yr
CRA II @ ICON: $100,000/yr
Comment below how much you get paid and what helped you get promoted!
Having a certification through CCRPS’s accredited Advanced Clinical Research Associate Certification course can help professionals 1) get promoted 2) get a raise 3) improve efficiency 4) get hired as a Clinical Research Associate.
Research Jobs Near Me
Clinical Research Jobs, Employment -
Clinical Research Positions and Job Opportunities
See CCRPS Remote, Entry-Level Clinical Research Job Bulletin
Clinical Research Positions and Job Opportunities
+7,000 Entry-Level Clinical Trial Jobs near me - Indeed
+7,000 Entry-Level Clinical Trial Jobs near me - Indeed
Google search “Clinical Research job role i.e. coordinator vs. associate vs. assistant near me since GOOGLE has a great job bulletin, simply google the job and set your email to get alerts on new openings
+16,000 Clinical Research Jobs near me- LinkedIn is a HIDDEN GEM for finding research jobs near me
Reaching out to CROs to express interest in their 1) trials 2) free intern roles is an option if location is a limiting factor (see ContractResearchMap)
Entry Level Research Jobs, Employment tips
Provide qualifications by getting a clinical research certification or degree. Consider enhancing your credentials with a Clinical Research Coordinator certification.
Modify resume to focus on clinical research and reframe past careers in how they could help your career in research (see VelvetJobs for 300+ clinical research resume examples)
Write focused cover letters for EACH job you apply to
Add anyone who emails you on LinkedIn
Follow up every 2-4 weeks until interviewed, hired, or rejected (must have rejection! this is how employers know your diligent and this sort of follow up shows immense interest in a large applicant pool).
How to get a research assistant job in the US as an IMG? - Follow the above steps! Share why your MD is useful in trials (detecting symptoms for patients and understanding enrollment criteria).
Research Remote Work From Home & Flexible Jobs - How to get a job doing research from home?
See our article on how to work remote in clinical research from home: 5 TIPS AND TRICKS TO WORKING FROM HOME IN CLINICAL RESEARCH DURING A PANDEMIC…OR REMOTELY. The COVID19 crisis has caused companies to require employees to work remotely in the clinical research field.
How to Get a Job as a Research Assistant
See our internationally-recognized, biopharma approved Clinical Trial Assistant Career Guide; viewed by thousands of visitors every month. The fastest way to prove both knowledge and passion in the field is to get certified. CCRPS Clinical Trials Assistant Training provides both ICH-GCP and senior-researcher level of content review so assistants can be both useful and an asset to the trial site!
Research Jobs
See our career guides and this great article by Kunal Sumpat on 15 Clinical Research Jobs
Clinical Research Associate (CRA) - How to become a clinical research associate? Start by getting a CRA certification.
Clinical Research Coordinator (CRC)
Drug Safety Monitor (PV) - Advance your career with a Pharmacovigilance Certification.
Clinical Trial Assistant (CTA) - How much does a research assistant earn?
Clinical Research Nurse (CRN)
All Research Professionals (ICH GCP)
Here's What You Need to Know to Get a Clinical Research Job - How to enter a research field?
This 5 hour video by Dan, Clinical Trial Guru, is a must-watch for all those interviews in clinical research jobs.
Argus Software - Oracle Argus Safety Database Training
Oracle Argus Safety
What is argus safety?
With the constant changing of FDA and International regulations surrounding drug safety = a need for a more adaptive and comprehensive drug safety software. Argus provides a strong technical ability to mine, report, and track safety data. Increased focus on a ‘duty of care’ through proactive safety management – rather than just meeting regulatory compliance. CCRPS offers pharmacovigilance training in oracle argus safety database.
Download the Oracle Argus Safety Training PDF here
Oracle Argus Training and Certification
Oracle Argus Safety Certification
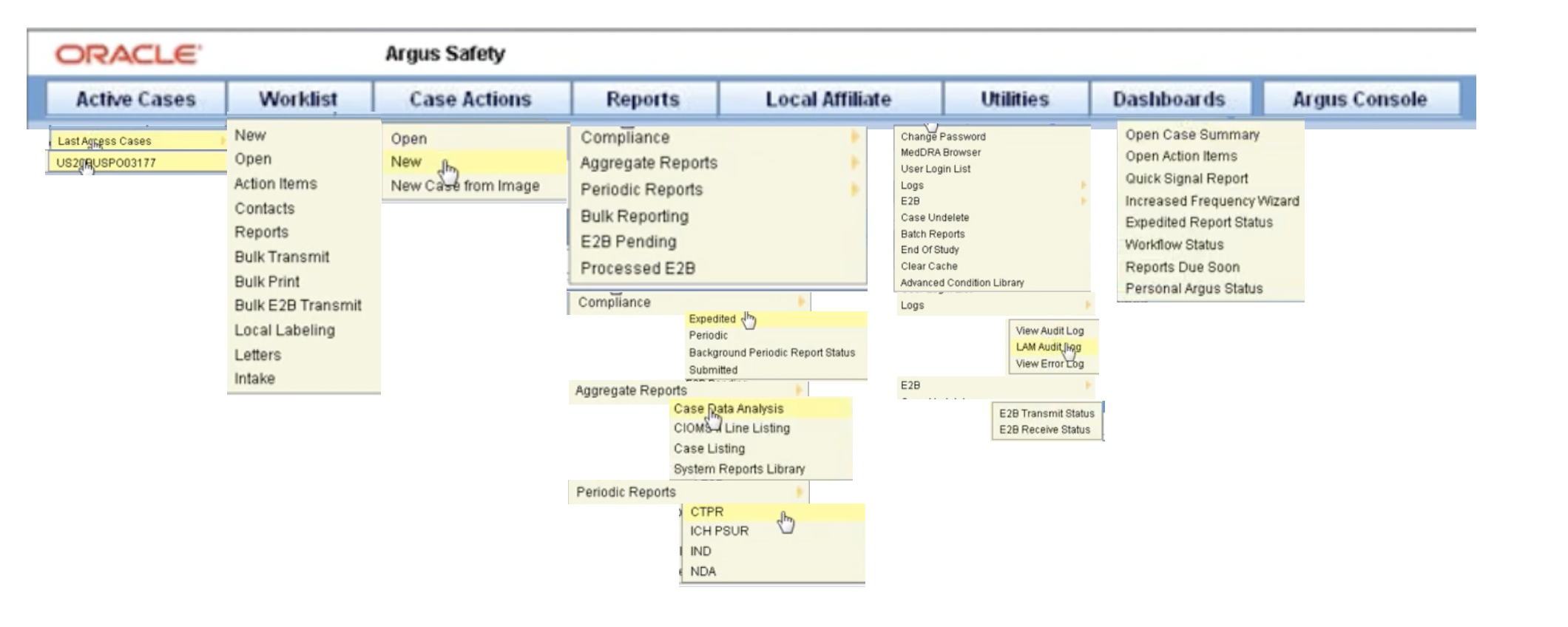
Oracle Argus Safety Certification is a 6-part program that covers every tab and task needed to manage drug safety data when using the Oracle Argus Safety Training application. See a skim-through of our Argus Safety Database training below. Our certification provides argus safety database training online which is internationally accepted for argus safety online training in the US, australia, bangalore, canada, europe, germany, mumbai, uk, and other major clinical trial-running countries.
Above, see our oracle argus safety database training with descriptions on how to use every indexed page and several application-focused references. Keep reading to see how argus clinical trials are conducted.
Who was Argus?
Argus was greek mythical giant with 100 eyes who was assigned by Zeus’ wife (Hera) to keep a vigilant watch of Zeus’s lover (Io) who had been turned into a cow.
Softwares used in Pharmacovigilance
While Oracle Argus Safety is the most-common software in safety monitoring, we’ve listed other common softwares you should quickly read up on (via google search of their user manuals) in case you come across them. FYI most Fortune 500 companies and CROs use Oracle Argus Safety as a preferred method of drug safety data management
Oracle Argus Safety
arisg safety database training
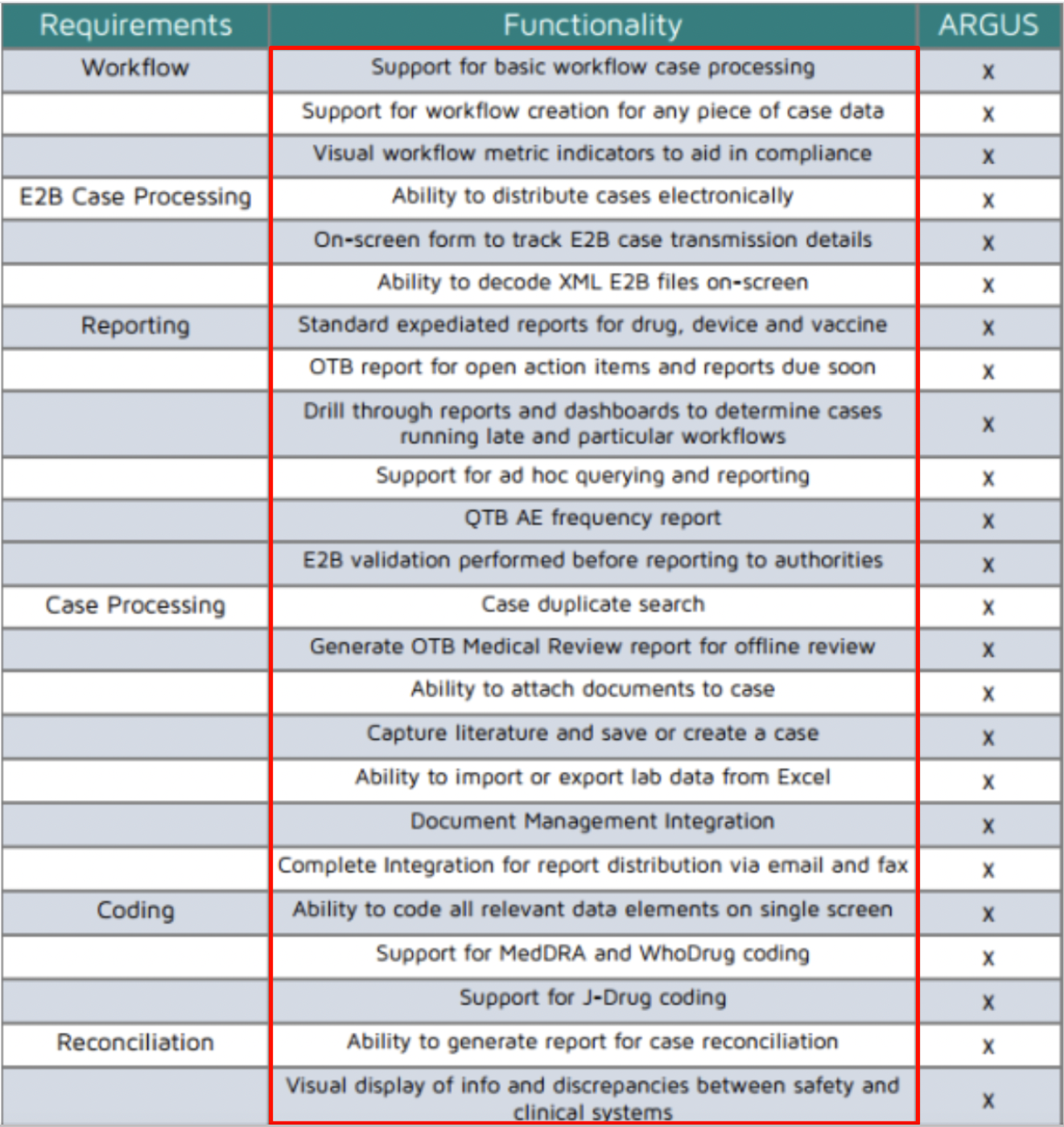
• The main essential features of Argus safety database include:
1. Supports all product types: Drugs, devices, vaccines, biologics, and gene therapies 2. Semi-Flexible configuration: Allows system to be tailored to your business needs
3. Built-in workflow: Migrate cases through workflow tasks
4. Complete audit history: 21 CFR Part 11 compliance
5. Integrated query module: Simple and complex analysis and surveillance
6. Report and submissions tracking: FDA/International expedited and periodic reports 7. Document Management: Attach any type of document
8. Dictionary Management: MedDRA and WHODrug coding and versioning
Oracle Argus Safety – navigation
Argus Drug Safety Training Free
Don’t need our certification or CME credits? Already training in PV/Drug safety? Already hired but just unsure of how to click through all those buttons? Trying to establish a more efficient flow in navigating argus drug safety database? Read below to get the “run-down” on how to teach yourself for free!
While certification helps boost the resume, most of us just need to learn what we need to do so we made a free training guide for everyone :).
Even without a argus safety certification course, students can trial the application themselves by downloading a free version of argus safety here. For argus safety database software free download see the Oracle® Argus Safety Installation Guide and download version 7.0 instead. FYI: Argus Safety installation requires a database first installed, to our knowledge version 7 is open-source to download so you can host argus safety.
Comment below if you have any trouble with this so our community can collaborate on seeing how individual demos can be set up for free real-world software training!
ORACLE Argus User Guide Links
Argus Suite: Argus Safety (Argus Affiliate, Argus Dossier, Argus Interchange, Argus Unblinding), Argus Safety Japan, Argus Insight, Argus Mart, Argus Analytics
Safety One Suite: Safety One Intake
Empirica Suite Empirica Signal and Topics, Empirica Study
Train yourself in Oracle Argus Safety by clicking on each user guide link here and making sure you understand the steps. Get access to a demo Oracle Argus Safety software here.
Know Your Basics: Log in, Change your password, Begin on the Personal Argus Status page, Use the quick launch toolbar, Use field validations, Acceptable date formats, Use other (than English) language text, Enter reasons for missing (null flavor) data (Video), Log out, Dynamic workflow indicator, Integrate with Argus Insight, Case form user preferences
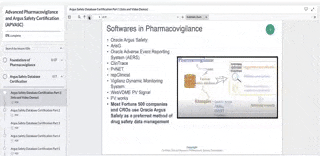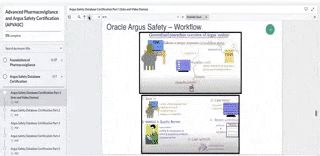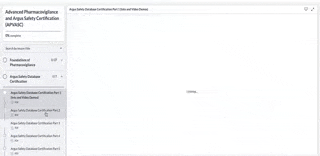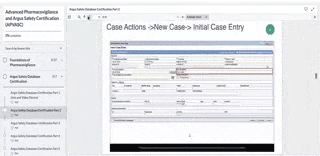
Enter Case Data: Create a new case (Video), Enter general information (Video), Enter patient information (Video), Enter products information (Video), Enter event information (Video), Add attachments to your case
Process Case Data: Access cases (Video), Process cases, Review cases (Video)
Filter Cases with Advanced Conditions: Create a single filter (Video), Create a set of related filters (Video), Share filters with other users, Modify a filter, Find filters, Use filters to view the case series list (Video), Use advanced condition library to access filter
Code an Adverse Event Term: Autocode a term, Manually code a term
Manage Your Expedited Report Submissions: Schedule an expedited report, Submit your expedited reports, Track your expedited report submissions, Manage your incoming ICSRs
Manage Your Periodic Report Submissions: Prepare content for periodic reports, View scheduled periodic report information, Submit a periodic report, Track your periodic report submission
Work in a Multi-tenant Argus Environment: Access your global worklist items
Configure Argus Settings: Manage access to Argus, Configure code lists, Configure products and licenses, Configure clinical studies, Configure reporting rules
Customize Your Reports Through BIP: Enable BI Publisher aggregate reporting, Configure the Argus BIP integration, Generate and modify a case series, Run a BIP periodic report through Argus Safety, View an aggregate report from Argus Safety, Argus Insight compatibility
Argus safety training videos:
This 4 hour argus safety lecture series we love (watch it, seeing someone go through the software once is more than enough training to start your job.)
Also see this wonderful argus drug safety training video lecture series on Oracle Argus Safety
Johnson and Johnson Vaccine Update (johnson and johnson vaccine)
Johnson and Johnson Covid Vaccine Update
6 similar and rare adverse events (cerebral sinus venous thrombosis i.e. blood clots in the veins of the brain) within 6-13 days in young-middle age women were reported after emergency release of the J&J covid vaccine used on 6.8m people so far (FDA Announcement)
Serious, Unexpected Adverse Event Case Study: Johnson and Johnson Vaccine
These JnJ covid vaccine adverse events were:
1) unexpected (not listed in the Johnson and Johnson vaccine trial/Janssen ENSEMBLE Clinical Trial Protocol or 2/21 Briefing document)
2) serious (resulting in death, hospitalization, etc)
What is Clinical Trials doing to protect patient safety?
3) The trial has paused so that causality can be found in order to determine if
a) the protocol needs to be amended to list this as an “expected” rare side effect
b) they need to discontinue the trial (to all or to specific demographics) if risk is greater than harm.
4) Causality is determined by physicians and researchers by assessing multiple factors including time of onset, biological possibility, confounders (i.e. patients predisposing conditions cause hypercoag state) strength of association (i.e. others getting higher rate of leg clots, etc), dechallenge (hyper-coagulability risk decreases after time).
5) After this, a root cause analysis to see if there are any confounders that could have caused these adverse events.
6) Then, a corrective action plan (CAPA) needs to be be created to help prevent this from happening again.
7) Last, documents like protocols would need to list this as an “expected’ event, however rare, and see if specific demographics (young to middle age women) are at risk and thus should not take it.
8) At this point, COVID itself still has a much higher risk of blood clots and thus getting an available vaccination (Pfizer, moderna) until more information regarding the Janssen Vaccine Adverse Event Causality is available is still key to protecting patient safety.
How do people report Vaccine Adverse Reactions?
9) Anyone is allowed to report vaccine adverse reactions online to VAERS (VAERS online reporting form; guide to reactions to report).
10) Anyone is allowed to view VAERS reports in excel format (2021 VAERS reports; search “Janssen” “covid vaccine” etc)
Share your thoughts below with us and let’s start a discussion to keep this article updated on the changes and new reports that come out regarding the ENSEMBLE trial.
7 Reasons Why You Should Start a Career in Clinical Research
begin a Career in Clinical Research
How you ever thought that there’s a perfect job out there for you, but you just haven’t found it yet? If you are motivated, informed, and interested in a science and medical career, you might have just found your answer. Many clinical research professionals say this is the golden ticket to a great career in the science field.
Why Clinical Research as a Career
The clinical research industry is a highly lucrative and expanding field. The global clinical trials market has been estimated at $46.8 billion in 2019.
As the push for new vaccines and therapeutics continues to get stronger, the field is expected to grow even more in value. Experts predict that the global market will hit $69.9 billion by 2027. The future in clinical research is bright, and it is one that you will want to be a part of.
Contrary to popular belief, working in clinical research doesn’t have to mean you have to stay in a lab. There are demands and opportunities for every skill set, if you know how to find them. Below, I have put together an in-depth guide on why you should get into clinical research.
Working in Clinical Research
You like a job that’s flexible
Don’t like working in a cubical? How about heading to the airport every morning instead? If you like a job that keeps you moving, then becoming a Clinical Research Associate (CRA) and working in clinical trials might be the right move for you. Learn more about becoming a CRA with this CRA Certification Course.
CRAs, contrary to what most people believe, don’t collect data or interact with patients. A CRA’s day-to-day job is to travel between different research sites and verify data transcription (i.e., data management). CRAs can also become part of the project management as a clinical trial manager of an entire trial.
They are also called “monitors” and a part of "regulatory affairs", because it is also their job and clinical experience to ensure that every site is following proper compliance and protocols.
There are two types of CRAs: home base or in-house. Home base CRAs work remotely. That means they work and travel from home. Most CRAs work for contract research organizations who are hired by sponsors to conduct their multi-site trials.
If you get tired of working home base, you can become an in-house CRA. In-house CRAs stay in one site and work together with a home base CRA to keep each other updated with what is happening at their site.
You like working with people
Have you ever been told that you are a people person with great communication skills? If talking to someone about how you can change their health for the better sounds like something you’d enjoy, you should definitely look into becoming a Clinical Research Coordinator (CRC). Explore our Clinical Research Coordinator Course to get started.
CRCs are the backbones to every project. They conduct patient visits, input source documents into the electronic data capture (EDC), and ensure that every trial is following compliance. They are also responsible for handling regulatory documents and updating the Principal Investigator (PI) with trial results.
CRCs conduct a variety of tasks, all of which impact the progress and development of the trial. Every successful clinical trials team needs is a good CRC. So, if you have strong interpersonal skills and know how to stay organized, you will be an indispensable part of the team.
You are detail-oriented and tech-savvy
Are you a self-proclaimed techie? Perhaps you’ve dabbled in coding, pick up computer programs easily, and maybe even have a background in IT. Technology is the future. If you think you have a knack for organizing data, you should look into becoming a Clinical Trial Assistant (CTA). Check out our Clinical Trials Assistant Training to learn more.
CTAs, also known as Clinical Research Assistants, manage the Trial Master File (TMF). They file, archive, and maintain trial documents and study files. They are also responsible for closing inquiries from the CRA, as well as providing administrative support to the research team. Every important step in clinical research, pre-clinical research, study startup, site management, needs a dependable CTA.
While most jobs in clinical research require some understanding of technology, it is especially important for the CTA to know what they are doing when it comes to managing trial documents and study files. In addition, it is equally important that the CTA is organized and knows how to pay attention to detail.
Working in Clinical Trials
Means you like a good salary...with room for promotion
Though there are many career paths within clinical research, most people begin their career as CTAs or CRCs in entry-level positions. Depending on your skill set and what kind of experiences you can bring to the table, either position will help you get your feet through the door.
According to salary.com, a CTA’s average salary in 2020 is $63,000. They generally earn between a range of $54,300 and $73,000, and are provided with benefits such as healthcare and social security.
If being more hands-on in the research process appeals to you, you might be a good fit for a CRC. Similarly, CRCs are making an average of $63,117 in 2020. Most make between a range of $54,210 and $72,902, plus employee benefits.
While numbers tend to fluctuate between cities and states, there’s no denying that these are great salaries for an entry position. Since according to one of the largest global job recruitment sites, Glassdoor, the average base salary in America is $40,000. Starting with an annual salary of $60,000 is considered uncommon and on the high end of the spectrum.
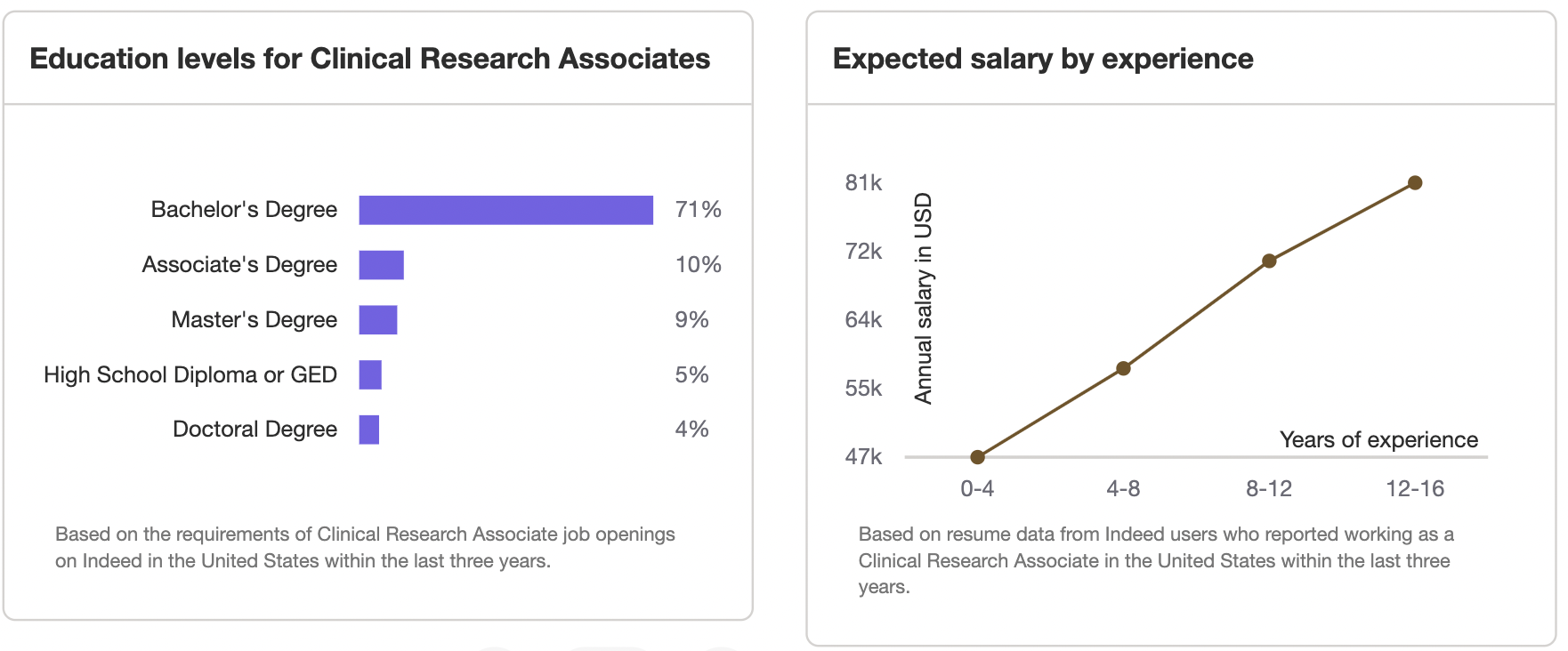
After one to two years of experience on the job, most companies provide CTAs and CRCs opportunities for professional development and promotion. Many become Clinical Research Associates, or CRAs. Indeed reports that the average salary of a CRA with one to two years of experience is $72,358. After building at least 6 years experience, a seasoned CRA should expect to earn $110,102 a year. If you would like to make more money, you can consider becoming an individual contractor CRAs. They can earn up to $300,000 in a year.
You are a science professional wanting to change careers and don’t want to go back to school
In clinical research, experience is often valued over degrees. Rather than what you didn’t study in college, hiring managers are more interested in what you have done in the past and how they can integrate you into their company.
This includes getting certified through clinical research courses, but more so what you learn from the courses you take. CCRPS offers the most in-depth CRA and CRC training so that there's tons to talk about during the interview and a working bank of knowledge during the first few months of the job. Explore more specialized certifications like ICH-GCP, Advanced Clinical Research Project Manager Certification, and Advanced Principal Investigator Physician Certification to further enhance your qualifications.
While graduate programs can help point you in the right direction, you don’t need a master’s degree to succeed in clinical research. In fact, certain positions don’t even require a bachelor’s or associate’s degree; they have certification in clinical research.
Applying to CRC and CTA positions are one of the most common segways into higher positions in clinical research. CRCs don’t need a bachelor’s or associate’s degree to get their foot in the door. While both CTA and CRA positions require a bachelor’s degree, they don’t have to be in the life sciences.
One of the best ways to gain experience and stand out from the crowd is to have on-site experience. If you need advice on how, Dan Sfera, the CEO of DSCS CRO Clinical Research Services, recommends getting started by interning or volunteering at clinics and research sites to build connections and experience.
Sometimes, the easiest way to get involved is to offer services like patient recruitment and social media management in exchange for opportunities to build your CV. By appealing to a site’s needs, this will help you get your foot in the door and build the connections and resume you need.
Another great way of adding experience to your resume is by training throughcertification courses. When employers see that you have taken the time and effort to understand how to be the best in their field, they are more far likely to hire you. At CCRPS.org, we offer seven courses and certification trainings to give you an advantage. Most of our students are hired within the first month of taking the course. We are accredited by the Accreditation Council For Clinical Research Education (ACCRE) and Joint Commission by the AMA, ANCC, and various other organizations to provide 17.5 CME credits through our CRA Certification and CRC Certification.
6. You are switching careers
Switching career fields can be nerve wracking. However, it is also an opportunity for you to be a unique candidate. Whether you come from a closely-related background, like medicine or nursing, or something completely different, there are ways you can advocate for yourself in front of employers.
If you already have a background in medicine (nonclinical doctor, unmatched MD), your knowledge of healthcare and your passion for patient health will make for a smooth translation into clinical research. In addition, your RN or MD degrees will help you gain a competitive edge and allow you to climb higher positions, such as the PI, who is the primary researcher of an operation.
On the other hand, if you come from a less relevant field but feel passionate, you can still leverage yourself to be exactly what the clinical research field needs. For example, if you are a teacher, your communication and interpersonal skills will be your keys to success. If you are a lawyer, your ability to draft and read papers will far surpass the average candidate.
If you studied mathematics, you are a skilled problem solver. If you are a translator, your language skills are valuable and will help you get into roles that require it. In short, whatever skills helped you succeed in your previous positions, you can bring it with you to clinical research.
7. You want to make a difference in disease outcomes and patient care
There are two types of people in the world: ones who accept the world as it is, and ones who strive to change it. In the last 50 years, science and medicine have gone through a series of drastic changes. However, anyone who works behind the scenes will tell you that medical breakthroughs are not miracles. Clinical research is the culmination of human effort and intelligence.
The fruits and labor of the ever-expanding industry are proof that if enough people care about the world, then they can change it. While there are many good reasons to work in clinical research, if you want the privilege to enrich the lives of others, there is a place for you in this field.
If you want to take a sneak peak at employers and opportunities near you, jobs sites like Indeed are a great resource.
Here are links for aspiring CRAs, for CRCs, and for CTAs. (Note: CTAs are often referred to Clinical Research Assistants, not to be confused for Clinical Research Associates)
Alternative Career Options for Doctors - Advanced Physician Medical Monitor Certification (APMMC)™
Clinical Research Physician Training
Triple-Accredited I 17.5 CME I 250 Hours I 100+ Modules I GCP Complaint I Instant Enrollment I 2+Wk Certification I
Alternative Career Options for Doctors:
Finding Your Dream Job in the US Healthcare System
For foreign doctors, navigating the path to practicing medicine in the US can be a long and challenging process. Each state has specific requirements, often including US residency training and USMLE certification. But what if you could utilize your medical knowledge and experience in another rewarding healthcare field?
Clinical Research: A Lucrative Opportunity
The world of clinical research is booming! The US Bureau of Labor Statistics predicts over 52,100 new jobs by 2024 https://www.bls.gov/ooh/fastest-growing.htm. This field is all about evaluating the safety and effectiveness of new treatments, drugs, and medical devices. Here's why it's perfect for foreign doctors:
Leverage Your Medical Degree: Your medical background gives you a significant head start in clinical research. Consider enrolling in our Clinical Research Coordinator or Clinical Trials Assistant Training courses to enhance your qualifications.
High Earning Potential: Experienced Medical Monitors can earn upwards of $300,000 annually (as of 2024 data). Ziprecruiter. For more specialized knowledge, consider our Medical Monitor Certification course.
Career Advancement: Your expertise opens doors to senior positions within the field. Boost your leadership skills with our Advanced Clinical Research Project Manager Certification or Advanced Principal Investigator Physician Certification.
Become a Pharmacovigilance Expert
Pharmacovigilance, or drug safety, focuses on monitoring and preventing adverse drug reactions. This rapidly growing field is ideal for those with a medical background due to:
Globalization of Pharmaceuticals: The expanding market creates a demand for professionals with diverse cultural and linguistic skills.
Increased Drug Use: Rising chronic illnesses lead to a greater need for drug safety specialists.
Competitive Salaries: According to 2024 data, the average annual salary is $145,231, with experienced professionals earning closer to $312,000.
Enhance your career by becoming certified through our Pharmacovigilance Certification course.
Clinical Data Science: Your Skills Translate to Success
Clinical data science involves extracting valuable insights from vast amounts of clinical data. It's a perfect fit for doctors with strong analytical skills and knowledge of clinical research protocols. Starting salaries average $97,000 (based on 2024 data), making it a lucrative career option.
Ready to Take the Next Step?
CCRPS offers comprehensive training programs to help foreign doctors like you transition into rewarding careers in clinical research. Explore our ICH-GCP and other courses to launch your exciting new path in the US healthcare system!
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
How to Be a Clinical Research Coordinator
Becoming a Clinical Research Coordinator Made Easy
A clinical research coordinator, often referred to as a clinical trial manager, plays a crucial role in all kinds of medical studies. The work of a clinical research coordinator is primarily under the primary investigator, who is inherently in charge of coordinating, designing, and facilitating the clinical trial in all aspects. If you are looking to step into this role, consider enrolling in a Clinical Research Coordinator course to gain the necessary skills and knowledge.
The job of a clinical research coordinator includes:
Ensuring all processes in the clinical trial are administered smoothly.
Building relationships with sponsors, departments, and institutions to meet the demands of the investigator.
Coordinating day-to-day activities under clinical trials to ensure smooth operations.
The Main Task of a Clinical Research Coordinator
To become a clinical research coordinator, understanding the roles and duties of the position is crucial. Key responsibilities include:
Planning and managing each experiment step to keep the administrative portion of the trial under control.
Enrolling and training initiatives under clinical research while adhering to relevant laws and regulations.
Designing experiments, managing research, and facilitating medical studies across all fields.
Enrolling and screening trial participants, implementing recruitment strategies, and maintaining relationships with all participants.
Securing approval from regulatory bodies and working within both laboratory and hospital environments.
For those interested in enhancing their understanding of good clinical practices, the ICH-GCP course is highly recommended.
Qualifications You Need
Becoming a clinical research coordinator requires:
A bachelor's degree in fields like microbiology, public health administration, or medical technology.
Preferably a master's degree, especially in management studies, due to the administrative and managerial nature of the role.
Completing specific courses, such as the Advanced Clinical Research Project Manager Certification, to enhance capabilities.
Popular courses for aspiring coordinators include biostatistics, biochemistry, epidemiology, mathematics, statistics, human anatomy, and healthcare management.
Skills You Need
The role of a clinical research coordinator is dynamic and requires various skills, including:
Management and communication skills, obtainable through specialized courses like Pharmacovigilance Certification and Medical Monitor Certification.
Interpersonal skills and enthusiasm for laboratory work and multitasking.
Another crucial step in building a career as a clinical research coordinator is gaining experience as an intern in healthcare or laboratory settings.
Becoming a clinical research coordinator is a great career choice for those motivated by a combination of medicine, management, and communication. Get started now for a bright future.
7 Steps To Becoming A Clinical Research Coordinator
7 Steps to Launching Your Career as a Clinical Research Coordinator
The prospect of a career in clinical research can be exciting, especially for those with a passion for science, medicine, and helping others. A Clinical Research Coordinator (CRC) plays a vital role in this field, ensuring research is conducted ethically and efficiently. If this sounds like the path for you, here are 7 essential steps to becoming a successful CRC:
Earn a Relevant Degree:
A bachelor's degree in a science-related field like biology, chemistry, or healthcare administration is typically required (National Institutes of Health .gov). Some employers may prefer a master's degree for more specialized roles (National Institutes of Health.gov). Consider exploring the Clinical Research Coordinator course for targeted training in this role.
Gain Hands-on Experience:
Internships or entry-level positions in clinical research settings offer invaluable experience (Association of Clinical Research Professionals (ACRP)). This practical exposure strengthens your resume and provides real-world knowledge for future CRC roles. Gain further insights through the Clinical Trials Assistant Training course.
Consider Certification:
While not always mandatory, CRC certification enhances your credentials and marketability. Programs like those offered by the ACRP validate your expertise and set you apart from other candidates. Expand your certification options with the CRA course and the ICH-GCP course.
Develop Core Skills:
Crucial skills for CRCs include: attention to detail, organization, critical thinking, and effective communication. A strong understanding of research regulations and ethics is also crucial. Enhance these skills through the Advanced Clinical Research Project Manager Certification.
Build Your Network:
Attend industry events, conferences, and seminars to connect with professionals. Networking opens doors to opportunities, mentorship, and valuable industry insights. Consider further specialization with the Advanced Principal Investigator Physician Certification.
Apply for CRC Positions:
With your qualifications, certifications, and experience in place, actively seek CRC positions. Tailor your resume to highlight relevant skills and experiences, and craft a compelling cover letter showcasing your passion for research. Research the organization and demonstrate your knowledge during interviews.
Embrace Continuous Learning:
The field of clinical research is constantly evolving. Stay informed about industry trends, participate in continuing education, and pursue professional development opportunities to stay ahead in your CRC career. The Pharmacovigilance Certification and the Medical Monitor Certification can be instrumental in your continuous learning journey.
References:
National Institutes of Health (.gov): https://toolkit.ncats.nih.gov/glossary/clinical-research-coordinator/
Association of Clinical Research Professionals (ACRP): https://acrpnet.org/
Society for Clinical Research Associates (SCRA): https://www.scra.org/
A description of Clinical Research Coordinator jobs and what they entail
Clinical research coordinators are usually supervised by clinical research managers. Their main task is to administer the clinical trials. Primary responsibilities normally include administering questionnaires, informing the participants about the objectives of the study, collecting data, and managing all the trials. They also have to adhere to all trial standards that have been set and also participate in recruitment of the subjects. Clinical research coordinators also have to engage with the subjects so that they can explain the things that are expected during the trial and also find out if they have any concerns. This means that a clinical research coordinator needs communicative and interpersonal skills.
For those interested in becoming clinical research coordinators, or enhancing their skills in this role, the Clinical Research Coordinator course provides comprehensive training and certification.
The responsibilities:
Maintaining records of all studies as per the guidelines.
Sticking to all ethical standards.
Sticking to all the regulatory standards set, including those covered in the ICH-GCP course.
Administering questionnaires.
Managing the budget dedicated to the research.
Overseeing the running of the trials as smoothly as possible.
Understanding and engaging with the subjects so as to know all issues.
Making sure that all equipment and supplies that are necessary for the success of the study are working and in stock.
Participating in the recruitment efforts of the participants, a topic extensively covered in the Clinical Trials Assistant Training.
Working with the laboratories so as to share findings.
Requirements:
The qualifications of a clinical research coordinator usually depend on your locations or employer. In most cases, for you to access clinical research coordinator jobs you should:
Have an associate nursing degree or any related field
Experience of two years within the healthcare industry
Analytical mindset
Be attentive to detail
Have interpersonal skills which are exceptional
Be ready to continue learning even without being prompted to do so, which can be further supported by the Advanced Clinical Research Project Manager Certification.
Great skills in organizing
Have great verbal and written communication skills
Additional certifications such as the Pharmacovigilance Certification, CRA, Advanced Principal Investigator Physician Certification, and Medical Monitor Certification are also beneficial for those looking to further their careers in clinical research.
Understanding what clinical research organizations are and what they do
CRO or a clinical research organization is a company which operates within the pharmaceutical industry in many cases. These kinds of organizations can be involved in many processes that are involved in the development of pharmaceuticals. There are also others who only have to administer required tests on drugs that are in development.
The large companies have their own clinical research organization incorporated into the structure of the company. There are yet others who outsource drug development and testing to the organizations which are precisely designed for such a purpose.
Why hire an independent organization
When you hire a clinical research organization which is independent to handle testing, the results are not highly doubted or questioned. This is because such an organization does not have any kind of self-interest in promoting something that is clearly bad. There have been cases where some medications tested by those who make them have failed to fulfill all promises made. However, clinical research organizations have shown more value to pharmaceutical companies.
Clinical research organizations are able to pave the way for chemicals that have been successful for approval by the relevant bodies. Most bodies have very high requirements and before approval, a lot of positive data have to be provided about the chemical. Clinical research organizations can assist with the supporting documents and the necessary paperwork so that approval can go through. Individuals interested in participating in this vital role might consider enrolling in a Clinical Research Coordinator course.
There are some concerns about when and where new drugs and chemicals are outsourced to the clinical research organization, but in most cases the concerns are of an economic standpoint. Sometimes the outsourcing of chemical research organizations that are not within your country may mean that scientists within the country will have lesser jobs.
Outsourcing these services saves a lot of money for pharmaceutical and chemical companies. When they do this, the company does not need to have a clinical department maintained within. This means less strain in HR departments. For those looking to deepen their expertise in the field, courses like Pharmacovigilance Certification, CRA, ICH-GCP, Clinical Trials Assistant Training, Advanced Clinical Research Project Manager Certification, and Advanced Principal Investigator Physician Certification are available.
Clinical Research Coordinator Certification
How To Get Clinical Coordinator Certification?
The clinical coordinator or CRC is also known as the clinical trial manager. They play an essential role in the studies of medicine of all types. They work typically under the personal investigator who has a charge of managing and conducting the clinical trial at a higher level. CRC's job is to facilitate, support, and to organize the daily trial activities of the clinical. Getting a clinical coordinator certification online will take a lengthy procedure. There are some kind of the CRC at very different phases of various educational achievements. Let's look at the steps which you will need to get the clinical coordinator certification.
Step 1: Must Be Graduate From High School (4 Years)
For preparing for the clinical coordinator certification, you must begin with high school courses like biology, chemistry, and physics, also with the maths and communications. This will found your form of the foundation which will be pursued for the college studies, which also be the four-year studies.
Step 2: - Obtain A Bachelor Degree Of 4 Years
When perusing the universities and the colleges, you must focus on the courses which offer you a bachelor's degree in health sciences in terms of clinical research administration. The students should dedicate them all the things to obtain this degree and must concentrate only on this for pursuing the degree full time. These programs generally provide the clinical research professional with the tools which they will need to do the developing their medical sciences and conducting their trials and also studies. Both on the campus and the online programs on the clinical administrations focus on clinical research for placing to protect the human subjects.
The bachelor's degree can give you an entry-level position in the clinical organization or any institution. It will also help you with the existing clinicians with the advance, which is in the current jobs. The students who wish to pursue more opportunities in terms of responsibility and also the salary might also be interested in specialized training such as the Clinical Trials Assistant Training or the Advanced Clinical Research Project Manager Certification.
Step 3: - Gain Some Professional Experience
In this step, you will need to gain some experience in the field. You will have to work in clinical research with full-time research. This is the requirements for qualifying the national clinical coordinator certification. For those looking to expand their knowledge in specific areas, consider the Advanced Principal Investigator Physician Certification or Medical Monitor Certification.
Step 4: -Obtain Online
In this step, the students can get an online graduate certificate in the clinical researcher, which can be of 1 year. This will help the students to grow some knowledge around the medical world. It will help the students for getting the clinical coordinator certification. Here you can explore many types of concepts of science and designing research, data management, professionalism, and leadership. This degree will offer you strong knowledge in clinical examination, ethical research and will also provide you the experience of the medical management skill related to drugs and other trials of therapeutic. For those interested in further certification, the ICH-GCP course is also available.
Step 5: - Online Master Of Science In Clinical Research Management
This online Master of Science course is done for increasing the salary purpose of the clinical research. This is a two years course. This course includes the clinical study of coordinators and as well as the data manager of the clinical, research assistants of the social science, and also about the clinical tech laboratories. Here you can explore many types of concepts of science and designing research, data management, professionalism, and leadership. After this course, the students will get some handsome salary and also be eligible for higher-grade jobs in clinical research. This will benefit you is pursuing the clinical coordinator certification. Those interested in Pharmacovigilance may consider the Pharmacovigilance Certification to enhance their expertise in this critical area.
Get The Certification
Now it's the time to hold the accreditation of your hard work, and it determines your success. There are some categories of certifications that will help you to understand which category you fall in.
Pharmaceutical and Contract Research Organization Employee Training
Pharmaceutical companies are confronting the increasingly-stressful challenge of providing higher performance at less cost and in less time whilst complying with an increasing amount of guidelines and regulations (like ICH GCP and the Clinical Trial Directive). This puts tremendous strain on the technical and technical skill foundation of all businesses, big, small or medium.
Certification courses, clinical study certain coaches and managers like CCRPS accountable for developing clinical study employees are now confronted with the requirement to deliver faster, powerful, and lasting coaching solutions. These solutions not just have to eliminate present gaps in ability, but in addition they will need to construct"World Class" competencies for clinical researchers.
In spite of the size of the business, resources could be restricted for spending on training clinical study staff. Hence, the investments in training have to get concentrated on providing invaluable training options that strategically enhance clinical research operation.
This restriction of funds may call out to the growth and skilful implementation of a pharmaceutical practice plan. This isn't only be a responsive solution where staff would register in publiclyavailable classes. Instead, it might entail building an inner training plan that would be strong and resilient in the face of daily pressures encountered while conducting clinical development applications. These pressures often overwhelm, and they occasionally wipe possible worth from off-the-job training, as employees may not have the capability to completely implement what they've learned.
This report asserts that a training plan is critical so as to maintain and harvest the complete value of crucial training interventions. Additionally, the training approach has to be appropriately sourced--maybe not the simplest, most evident solution available on the industry.
A"Training Plan" could be described as value-added, prioritized and readily-implementable strategy for addressing both present and future performance openings.
A training approach links the organizational and business goals with the requirements of people. The training plan has to be dynamic so as to account for modifications inside the business in addition to external changes (e.g., the rising regulations impacting drug development like the Clinical Trial Directive, along with also the ICH electronic common technical document, and raising Phamacovigilance needs ).
The components of the definition could be dissected as follows:
Value-added -- The coaching plan should Not Just address the Particular performance enhancements that will be achieved, but it should also provide a business case because of its rationale (e.g., the coaching intervention Ought to Be aligned with the clinical trial program development strategy )
Prioritized -- The plan should Take into Consideration comparative beauty, difficulty of execution, and urgency
Implementable -- It Ought to Be carefully examined against probable obstacles to bringing its Entire worth
Strategies -- A coherent Summary of Important goals, options and activities (the"how"), ought to be provided
Thorough -- The coaching plan shouldn't be curative -- it ought to also address Substantial improvements in coaching abilities, in Addition to differences between current skills and new abilities needed to meet challenges in the future surroundings
Before we consider creating a training plan, let's analyze its possible additional value.
"Worth" originates in the notion of'economic value'; finally, all company activities have a stage to indirectly or directly create income stream. Even worth of company staff comes from using growth capacity and safety, and it is ultimately linked to the cash flow.
Coaching from an assortment of resources ends in potentially-increased financial price.
In clinical study, this comprises:
Insightful and synergistic conclusion in medication development (e.g., growth of a lifestyle medication such as Viagra)
Avoiding mistakes, disturbance and re-work (e.g., powerful observation for the reduction of Information inquiries )
Accelerating procedures (e.g., quicker patient recruiting via CRAs, more efficiently motivating investigator site groups )
Enhancing the adoption of specialized know-how from the execution of an electronic data capture system and a clinical trial management system
Aligning behavior (e.g., clinical research team and information management understanding each other's needs to Allow them to create CFRs that facilitate more precise data collection, in Addition to enabling easy data entry)
Shifting mind-set (e.g., clinical study team welcoming audit and review as an Chance to verify the GCP compliance of the procedures )
Every one of those value-creating actions is targeted concerning this'From-To's' of changes -- that will ultimately create economic price. This is sometimes expressed as a"From-To" or"FT" evaluation. This analysis may be used to assess the difference in experience in a skills area so as to think about how to handle the gap and to help evaluate the efficacy of the coaching intervention.
Some pharmaceutical companies have started to evaluate the company value of implementing training plans. This value-based mind-set isalso, possibly, brand new to pharmaceutical firms and also to those responsible for executing clinical research training. Basically, the vital ingredient for every single training initiative would be to get a business case that about aims its worth.
Diagnosis of present position generally starts with some concentrated competency (skills) evaluation. Competency analysis entails collecting data and can easily grow to be a significant exercise, one at risk of having an end in itself. This is very true in the pharmaceutical sector, in which there are lots of technical specialties.
An excellent starting point would be to examine the applicable current clinical study processes to recognize the main clinical study project skills, job skills, scientific/technical and social abilities. A variety of approaches may be utilized to collect data, such as structured interviews, surveys, focus discussion groups, audit findings, and formal or informal opinions including advice from surveys regarding training requirements. You don't have to use these methods and frequently, a sample of structured interviews of the two supervisors and staff regarding a specific job function provides quite informative information, because this is an especially flexible method of getting insights. Individuals are not as inclined to offer insights when just answering a typical questionnaire.
The information gathered will be assessed and examined to permit you to assess the first difference between the current proficiency and the skills demanded of a medical study regimen. A first training gap analysis could be attracted by differentiating between the following:
Where you reside, and where you Have to Be currently
Where you reside, and where you Have to Be in the near - to become what is known as"World Class" in clinical study
A report can then be made to explain the goal of the particular training needs evaluation, the processes used to accumulate the information, the abilities / performance openings and original options for handling these requirements. Some of those needs will be particular training options and others will probably be types of organizational aid, for example performance management.
Ideally, an individual will be"World Class" in all concerning clinical research in addition to in fixing all of the gaps in skills identified by the training needs analysis. Not only is this unrealistic, it might also bring about your dispersing of coaching tools much too .
Since the training gap analysis is very likely to spot a high number of training requirements, it's extremely beneficial to prioritize the areas to be dealt with in order to maximize effectiveness. It's helpful to evaluate their potential beauty and execution difficulty when taking out an initial prioritization of coaching proficiency gaps. This may be achieved with a technique known as the attractiveness-implementation problem grid (or'AID' evaluation ) -- consult for a number of examples of potential training openings. This output will fluctuate based on the specific findings of this training gap analysis, and won't be the exact same for many businesses.
Here, it's invaluable to distinguish between constant improvements (which involve maintenance of present capacity, or moderate improvements of it) plus a significant change in capacity which will have a significant effect on present and future operation.
A good illustration of a significant pharmaceutical abilities change is that over the past couple of decades, contract research organisations (CROs) have recognized they will need to invest around six months on intensive training to equip new untrained tracks together with the abilities to track clinical trials before they're effective at tracking sites independently. This has allowed CROs to fulfill the pharmaceutical firms with the idea that they'll attain sufficient quality criteria for tracking.
The region of digital data capture (EDC) introduces another instance where some businesses are wanting to conduct 90 percent of the clinical trials using EDC so as to lower the opportunity to enter clinical trial information. This is a significant shift in clinical research and investigative personnel work. For EDC implementation to work, important training initiatives are essential to ease changes in data collection procedures.
A substantial shift that the industry is researching is that the development of management skills in clinical study global clinical trial applications. Also, the business is analyzing clinical research for coaching the rising amount of clinical trial administrators, understanding management changes in regulatory conditions, and clinical study certification.
Not many significant training initiatives could be tried concurrently. Observing the Japanese doctrine of breakthrough, or"HOSHIN", it's ideal to employ only between one and three simultaneously, particularly at the onset of implementation. By shocking the successful execution of big training initiatives with time, maybe five or six goals might be fulfilled in 18-24 months.
After lots of tactical training priorities are identified, it's vital to examine their crucial interdependencies. All too frequently, training initiatives exist because comparatively self explanatory initiatives, while their potential and full value is highly dependent upon many of actively-managed interdependencies. Reaching full alignment of those interdependencies requires significant placement and communication of every initiative together with the players involved. It's vital to use an additional visual method, for example comprehensive stakeholder analysis. Here we view stakeholders (who may incorporate decision makers, advisers, implementers, or recipients), examined with their various approaches ('for','impartial' or'against') and their degree of influence.
In my experience, each the analytical methods covered in this guide must become involved so as to develop an effective training plan. The vital training required in important areas of"extend" and areas requiring continuous improvement ought to be identified together with the entire participation of senior line managers. The training section must act as the expert advisor on how instruction issues would be best addressed, implemented and tracked. However, this function shouldn't wholly or mostly take over the coaching intervention.
Besides getting the possession of senior management in the start, it's highly recommended that management is included in the comprehensive planning, the execution of their training programs, and also studying the support procedures themselves.
Reader exercise
To what extent does the version of the numerous functions (as described previously ) reflect practices in your business?
Where this doesn't occur, and what will be the issues and costs arising because of this?
Does line direction now have the abilities and mind-set to achieve this position? (Otherwise, what support and training are needed?)
Can the Training Department now have the abilities, mind-set and authenticity to accomplish this? (Otherwise, what training tools and repositioning are required and if these be sought externally, either in part or whole?)
Unless There's a close and genuinely symbiotic relationship between senior management and the coaching section, then the default option training plan likely to be implemented could be distinguished as follows:
A center of regular, routine in-house coaching programs which tackle past training demands -- partly and as one-on-one classes without actual followup and follow-through
The value/ROI (return on investment) of instruction is often assessed chiefly by"satisfaction sheets" instead of by real, concrete value-added evaluation
New requirements are characterized by senior management, and therefore are often not well identified. The Training Department/ coaching function is forecast to respond fast to place something in place, which occasionally fails to provide alternatives
Line supervisors and the coaching department/training function are responsive to individual requests for coaching, the outcome being that a lot of emphasis will be put on more costly and untailored public applications. When people return to their businesses, the learning dissipates as a consequence of the business's unchanged mindset.
This brings us to the problem of sourcing your coaching plan.
Possibly the ideal mix for coaching is that of tailored inner classes with follow-on job work and discerning mentoring alongside a reduce dependence on conventional classes such as predictable, core skills coaching.
A tailored, in-company strategy is especially suited for:
Technical abilities, if these are firm or department-specific
Job management abilities
General management abilities - like"people skills," strategic thinking, change direction, etc..
Direction and team-building
A good illustration of a tailored in-course program that combines the greatest internal and external firm standpoint and addresses a significant place where clinical study supervisors would help in enhancing their management abilities is titled,"World Class Management Skills - The illustration of the Mini Pharma MBA."
Combining Internal and External Trainers
An extremely strong shipping mix will be to supply tailored programs having an inner training resource and an outside supplier who will incorporate an external perspective. The Selection of an outside supplier could be based on the following criteria:
Technical skill in clinical research and GCP, monitor record and credibility of coaching clinical research and GCP QA professionals
Training abilities and fashion
Willingness to Know the company and provide a trulytailored training alternative
Probably fit inside the Organization's civilization
Flexibility and responsiveness
Concentrate on actual value-added (instead of simply on coaching and finishing satisfaction polls )
Conclusion and Next Steps
Pharmaceutical companies are placing ever-increasing requirements on their employees to execute to"World Class" criteria of clinical investigation, and this also necessitates the development and execution of a successful Training Strategy, using the above arrangement of identification, creation, and execution. Creating a really robust approach demands the symbiotic job of senior managers and a business-focused, strategic instruction section.
The training plan should have lots of crucial (prioritized) significant training initiatives during its heart. All these ought to be diagnosed with line supervisors, be well set up, and should subsequently be sourced in a innovative method to satisfy needs. Training options should be tailored as you can, preferably without emphasis on public classes and standardized internal classes.
Creating a training plan is a fascinating frontier for bringing clinical study into accordance with world class administration. Is your business prepared for the question?
Clinical Research Associate Training UK
Clinical research is a field with a lot of career potential all over the world. Clinical research associates, or CRAs, are one of the most sought-after positions in the field. CRAs monitor labs and research processes. It is a position that offers financial security and many traveling opportunities for work. In this article, we have put together a short work guide on becoming a CRA in the UK. According to SalaryExplorer, the 2020 average salary of a CRA in the UK is 82,700 GBP. Depending on your experience and location, you can expect to earn anything from 38,000 GBP to 131,000 GBP.
United Kingdom Clinical Research Career Guide
Clinical research is a field with a lot of career potential all over the world. Clinical research associates, or CRAs, are one of the most sought-after positions in the field. CRAs monitor labs and research processes. It is a position that offers financial security and many traveling opportunities for work. In this article, we have put together a short work guide on becoming a CRA in the UK. For more detailed training, check out our CRA training program.
Salary:
According to SalaryExplorer, the 2020 average salary of a CRA in the UK is 82,700 GBP. Depending on your experience and location, you can expect to earn anything from 38,000 GBP to 131,000 GBP.
On average, CRAs with less than 2 years of experience earn an average salary of 43,200 GBP. However, a CRA’s salary increase exponentially after 2-5 years of experience, increasing by 34% (57,700 GBP per year). They increase another 48% after obtaining 5-10 years of experience (85,200 GBP per year).
Requirements to Work:
The minimum education requirement to work as a CRA in the UK is a bachelor’s degree. However, investing in a master’s degree will increase your qualifications and earning power. SalaryExplorer has shown that CRAs with a master’s degree have a 93% higher average salary than CRAs with just a bachelor’s degree. Explore our ICH-GCP course to enhance your qualifications.
Although clinical research is a global field, job requirements and salaries can differ from place to place. After reading this guide, we hope that you have a better understanding of what it is like to work in the UK. If you want to learn more about clinical research and becoming a CRA, please check out our CRA training program and our articles below to learn more about the international job scene.
The aim of this course is to help healthcare professionals and other non-medical/dental healthcare professionals and those that are already working in the clinical research industry who want to develop and acquire skills and knowledge. This course will help you to develop the knowledge of the theoretical aspects of clinical research and the skills that are relevant in research methodology that are applicable to researches in the contemporary clinical practice settings.
The benefits of taking this clinical research online course are:
It helps you to develop an in-depth knowledge and an understanding of the nature, purpose, different methods, and application of research that are relevant to clinical practice as an individual or in an organization.
It develops and builds your capacity for research and fosters evidence-based practice. This is done by equipping you with the knowledge and skills that are needed in the critical appraisal, application, designing, and undertaking of high-quality research within different range of clinical settings.
It provides you with an excellent research training with outstanding resources and expertise in the field, in a supportive environment that allows you to thrive in your pursuit for clinical research knowledge.
It will enhance your opportunity to pursue a career in clinical research and an opportunity to further your clinical research training with advanced knowledge of clinical research and practice. Consider our Advanced Clinical Research Project Manager Certification and Advanced Principal Investigator Physician Certification.
It will develop your critical thinking and reflection skills, help you to be more effective in communication, improve your team and multidisciplinary work, your use of health informatics, and help you to be more systematic in decision-making and problem-solving.
You will have the opportunity to learn from and interact with renowned professionals from the clinical research field, and other health-related fields, and other industry experts who will serve as lecturers for this course.
This mode of this clinical research online program allows you to be more flexible with how you learn. The proprietor ccrps.org makes use of digital technology such as individual and group web based audiovisual tutorials and discussion boards in order to ensure the supervision of their students and constant communication. We are here to ensure that students meet the field standards and that the students are able to learn.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
A Comprehensive Guide to Clinical Research Careers in Switzerland
High-Paying Career with Travel Opportunities Awaits!
The world of clinical research offers a wealth of exciting career paths, and one of the most sought-after roles is the Clinical Research Associate (CRA). CRAs play a critical role in ensuring research is conducted smoothly and ethically, overseeing lab activities and research procedures. Not only does this career offer financial stability, but it can also open doors to travel-based work experiences.
This comprehensive guide explores everything you need to know about becoming a CRA in Switzerland in 2024.
How Much Can You Earn?
Switzerland offers competitive salaries for CRAs. According to SalaryExpert: the average CRA salary in Switzerland was CHF 149,000 in 2020. This can vary depending on your experience and location, with a range of CHF 68,700 to CHF 237,000.
Entry-level CRAs (less than 2 years of experience) typically earn around CHF 78,000 annually.
With 2-5 years of experience, salaries jump significantly by 34% to an average of CHF 104,000 per year.
CRAs with 5-10 years of experience see another increase of 48%, reaching an average annual salary of CHF 154,000.
What Qualifications Do You Need?
In Switzerland, a bachelor's degree is the minimum requirement to become a CRA. However, a master's degree can significantly boost your qualifications and earning potential. SalaryExpert data shows that CRAs with a master's degree earn an average 93% more than those with only a bachelor's degree.
Standing Out in the Swiss Market
Clinical research is a global field, but job requirements and compensation can differ by region. This guide provides insights specifically for the Swiss market.
Ready to Launch Your CRA Career?
If you're passionate about clinical research and interested in becoming a CRA, explore our CRA training program to gain the necessary skills and knowledge. Additionally, check out our courses on Clinical Research Coordinator, Pharmacovigilance Certification, and ICH-GCP to further enhance your qualifications.
Serbia Clinical Research Career Guide
Serbia Clinical Research Career Guide
Clinical research is a field with a lot of career potential all over the world. Clinical research associates, or CRAs, are one of the most sought-after positions in the field. CRAs monitor labs and research processes. It is a position that offers financial security and many traveling opportunities for work. In this article, we have put together a short work guide on becoming a CRA in Serbia.
1. Clinical Research Associate (CRA) Salary
Clinical Research Associate (CRA) Salary
Average Monthly Salary (2024) According to SalaryExplorer: As of 2024, the average monthly salary for a Clinical Research Associate (CRA) in Serbia is 156,000 RSD. Depending on your experience and location, you can expect to earn anything from 81,200 RSD per month to 239,000 RSD per month.
Experience-Based Trends:
Less Than 2 Years of Experience: CRAs with less than 2 years of experience earn an average of 92,200 RSD per month.
2-5 Years of Experience: After 2-5 years of experience, salaries increase significantly by 32%, reaching an average of 124,000 RSD per month.
5-10 Years of Experience: With 5-10 years of experience, CRAs see another substantial increase of 36%, bringing their average salary to 161,000 RSD per month.
2. Education and Earning Power
Minimum Requirement: To work as a CRA in Serbia, a bachelor’s degree is the minimum educational requirement.
Master’s Degree Advantage: Investing in a master’s degree can significantly boost your qualifications and earning potential. CRAs with a master’s degree earn an average salary 39% higher than those with only a bachelor’s degree.
Relevant Courses: If you're considering advancing your career in clinical research, you may find these courses beneficial:
Clinical Research Coordinator: Course Link
CRA: Course Link
ICH-GCP: Course Link
3. Serbia’s Evolving Research Landscape
Recent Salary Changes: In January 2024, Serbia increased the salaries of its top 10% of scientists. While this specific data doesn’t directly apply to CRAs, it reflects the country’s commitment to supporting research professionals.
Requirements to Work:
The minimum education requirement to work as a CRA in Serbia is a bachelor’s degree. However, investing in a master’s degree will increase your qualifications and earning power. SalaryExplorer has shown that CRAs with a master’s degree have a 39% higher average salary than CRAs with just a bachelor’s degree.
Further Learning Opportunities: If you're interested in advancing your knowledge and skills in clinical research, consider exploring our additional courses:
Pharmacovigilance Certification: Course Link
Clinical Trials Assistant Training: Course Link
Advanced Clinical Research Project Manager Certification: Course Link
Advanced Principal Investigator Physician Certification: Course Link
Medical Monitor Certification: Course Link
Although clinical research is a global field, job requirements and salaries can differ from place to place. After reading this guide, we hope that you have a better understanding of what it is like to work in Serbia. If you want to learn more about clinical research and becoming a CRA, please check out our CRA training program and our articles below to learn more about the international job scene.
Norway Clinical Research Career Guide
Clinical research is a field with a lot of career potential all over the world. Clinical research associates, or CRAs, are one of the most sought-after positions in the field. CRAs monitor labs and research processes. It is a position that offers financial security and many traveling opportunities for work. In this article, we have put together a short work guide on becoming a CRA in Norway.
1. Clinical Research Associate (CRA) Salary
Clinical Research Associate (CRA) Salary
Average Monthly Salary (2020): As of 2024, the average monthly salary for a Clinical Research Associate (CRA) in Norway is 684,000 NOK. Depending on your experience and location, you can expect to earn anything from 314,000 NOK per month to 1,090,000 NOK per month.
Experience-Based Trends:
Less Than 2 Years of Experience: CRAs with less than 2 years of experience earn an average of 357,000 NOK per year.
2-5 Years of Experience: After 2-5 years of experience, salaries increase significantly by 34%, reaching an average of 477,000 NOK per year.
5-10 Years of Experience: With 5-10 years of experience, CRAs see another substantial increase of 48%, bringing their average salary to 705,000 NOK per year.
Link Integration: No specific section for course links. However, considering the emphasis on the role of a Clinical Research Associate, we can integrate relevant course links here.
You may want to enhance your skills and qualifications for a successful career as a Clinical Research Associate. Check out our CRA course to gain comprehensive training in this field.
Education and Earning Power
Minimum Requirement: To work as a CRA in Norway, a bachelor’s degree is the minimum educational requirement.
Master’s Degree Advantage: Investing in a master’s degree can significantly boost your qualifications and earning potential. CRAs with a master’s degree earn an average salary 39% higher than those with only a bachelor’s degree.
Link Integration: We can integrate the course links here to emphasize the importance of education and training for career advancement.
Considering the significance of education in enhancing your career prospects, explore our courses such as Clinical Research Coordinator and Pharmacovigilance Certification to broaden your knowledge and skills in clinical research.
Conclusion
A clinical research career in Norway offers exciting prospects. Combine education, practical experience, and a passion for advancing medical science to contribute significantly to patient outcomes. Whether you’re just starting or have years of experience, Norway’s clinical research field awaits your expertise! 🌟
Link Integration: Let's reinforce the importance of education and training in clinical research by adding relevant course links here.
To embark on a successful journey in clinical research, consider enrolling in courses like ICH-GCP and Clinical Trials Assistant Training to equip yourself with essential knowledge and skills.
Macedonia Clinical Research Career Guide
Clinical research is a field with a lot of career potential all over the world. Clinical research associates, or CRAs, are one of the most sought-after positions in the field. CRAs monitor labs and research processes. It is a position that offers financial security and many traveling opportunities for work. In this article, we have put together a short work guide on becoming a CRA in North Macedonia.
Salary:
Based on the latest 2024 data, the average salary for a Clinical Research Associate (CRA) in North Macedonia has likely changed since 2020. While specific updated figures for CRAs are not provided, the overall average salary in Macedonia is reported to be 1,360,113 MKD per year1. This suggests that there might have been an increase in the average salaries across different professions, including CRAs.
Given the general salary growth, CRAs in North Macedonia with less than 2 years of experience, who previously earned an average of 32,000 MKD a month, may now earn more. Similarly, the salary increments after 2 years of experience and again after 5-10 years of experience would also have likely increased proportionally.
For the most accurate and updated figures for CRA salaries in North Macedonia, it’s best to consult the latest reports or salary surveys that are specific to the field of clinical research. These resources will provide current information, taking into account factors such as experience, location, and education level.
Here’s an updated summary based on the 2024 data:
The average annual salary in Macedonia is 1,360,113 MKD.
Salaries vary between genders, with men receiving an average of 1,451,025 MKD and women 1,177,563 MKD.
The highest-paid careers are Engineers & Technicians III and Architect Constructions, with average incomes of 7,065,379 MKD and 3,270,389 MKD, respectively.
Education impacts earnings, with those holding a Master’s Degree earning an average of 1,746,811 MKD and those with a Doctorate Degree 1,674,260 MKD.
Experience also affects salaries, with individuals having 20+ years of experience earning 2,182,119 MKD, and those with 16-20 years of experience 1,981,208 MKD.
For a detailed update on CRA salaries, please refer to the latest specialized salary surveys for clinical research professionals.
Requirements to Work:
The minimum education requirement to work as a CRA in North Macedonia is a bachelor’s degree. However, investing in a master’s degree will increase your qualifications and earning power. SalaryExplorer has shown that CRAs with a master’s degree have a 39% higher average salary than CRAs with just a bachelor’s degree.
Although clinical research is a global field, job requirements and salaries can differ from place to place. After reading this guide, we hope that you have a better understanding of what it is like to work in North Macedonia. If you want to learn more about clinical research and becoming a CRA, please check out our CRA training program and our articles below to learn more about the international job scene.
Explore Our Comprehensive CRA Training Program for Career Advancement in Clinical Research
Course Links:
Clinical Research Coordinator: https://app.ccrps.org/courses/Clinical-Research-Coordinator
Pharmacovigilance Certification: https://app.ccrps.org/courses/pharmacovigilance-certification
Clinical Trials Assistant Training: https://app.ccrps.org/courses/Clinical-Trials-Assistant-Training
Advanced Clinical Research Project Manager Certification: https://app.ccrps.org/courses/Advanced-Clinical-Research-Project-Manager-Certification
Advanced Principal Investigator Physician Certification: https://app.ccrps.org/courses/Advanced-Principal-Investigator-Physician-Certification
Medical Monitor Certification: https://app.ccrps.org/courses/medial-monitor-certification
Explore our comprehensive CRA training program to take your career in clinical research to new heights. Our courses cover essential topics and provide practical skills to excel in the field of clinical research. Whether you're starting your journey as a Clinical Research Associate or looking to advance your career, our specialized training programs cater to professionals at every level. Enroll today and unlock exciting opportunities in the dynamic field of clinical research!
CROs:
Bosnia and Herzegovina Clinical Research Career Guide
Bosnia and Herzegovina Clinical Research Career Guide
Clinical research is a field with a lot of career potential all over the world. Clinical research associates, or CRAs, are one of the most sought-after positions in the field. CRAs monitor labs and research processes. It is a position that offers financial security and many traveling opportunities for work. In this article, we have put together a short work guide on becoming a CRA in Bosnia and Herzegovina.
Salary:
According to SalaryExplorer, the average monthly salary of a Clinical Research Associate (CRA) in Bosnia and Herzegovina is between 1,154 BAM and 2,835 BAM in 2024. Here’s a breakdown based on experience:
Less than 2 years of experience: On average, CRAs with less than 2 years of experience earn around 1,420 BAM per month.
2-5 years of experience: After gaining 2-5 years of experience, a CRA’s salary increases significantly by 33%, reaching an average of 1,890 BAM per month.
5-10 years of experience: With 5-10 years of experience, CRAs see another substantial increase of 48%, bringing their average monthly salary to 2,790 BAM.
Keep in mind that actual salaries may vary based on your specific experience, location, and other factors. It’s always a good idea to research local market trends and negotiate your compensation accordingly.
Elevate Your Career in Clinical Research with Our CRA Training Program
Clinical Research Coordinator: https://app.ccrps.org/courses/Clinical-Research-Coordinator
Pharmacovigilance Certification: https://app.ccrps.org/courses/pharmacovigilance-certification
Clinical Trials Assistant Training: https://app.ccrps.org/courses/Clinical-Trials-Assistant-Training
Advanced Clinical Research Project Manager Certification: https://app.ccrps.org/courses/Advanced-Clinical-Research-Project-Manager-Certification
Advanced Principal Investigator Physician Certification: https://app.ccrps.org/courses/Advanced-Principal-Investigator-Physician-Certification
Medical Monitor Certification: https://app.ccrps.org/courses/medial-monitor-certification
Requirements to Work:
The minimum education requirement to work as a CRA in Bosnia and Herzegovina is a bachelor’s degree. However, investing in a master’s degree will increase your qualifications and earning power. SalaryExplorer has shown that CRAs with a master’s degree have a 93% higher average salary than CRAs with just a bachelor’s degree.
Although clinical research is a global field, job requirements and salaries can differ from place to place. After reading this guide, we hope that you have a better understanding of what it is like to work in Bosnia and Herzegovina. If you want to learn more about clinical research and becoming a CRA, please check out our CRA training program and our articles below to learn more about the international job scene.
Albania Clinical Research Work Guide
Clinical research is a field with great career potential all over the world. Clinical research associates, or CRAs, are one of the most sought-after positions in the field. CRAs monitor labs and research processes. It is a position that offers financial security and many traveling opportunities for work. In this article, we have put together a short work guide on becoming a CRA in Albania.
Salary:
According to SalaryExplorer, the average monthly salary of a Clinical Research Associate (CRA) in Albania in 2024 is between 47,420 ALL and 131,405 ALL1. Let’s break it down based on experience:
Less than 2 years of experience: On average, CRAs with less than 2 years of experience earn around 60,000 ALL per month.
2-5 years of experience: After gaining 2-5 years of experience, a CRA’s average salary increases by 34%, reaching an average of 80,100 ALL per month.
5-10 years of experience: With 5-10 years of experience, CRAs see another substantial increase of 48%, bringing their average monthly salary to 118,000 ALL.
Remember that actual salaries may vary based on your specific experience, location, and other factors. It’s always wise to research local market trends and negotiate your compensation accordingly. 🌟
For more detailed information, you can explore the SalaryExplorer page for Clinical Research Associates in Albania.
Requirements to Work:
The minimum education requirement to work as a CRA in Albania is a bachelor’s degree. However, investing in a master’s degree and furthering your education increases your qualifications and earning power. For example, SalaryExplorer has shown that CRAs with a master’s degree have a 93% higher average salary than CRAs with just a bachelor’s degree.
Although clinical research is a global field, job requirements and salaries can differ from place to place. After reading this guide, we hope that you have a better understanding of what it is like to work in Albania. If you want to learn more about clinical research and becoming a CRA, please check out our CRA training program and our articles below to learn more about the international job scene.
Explore Courses for Clinical Research Careers in Albania
Courses Available:
Montenegro Clinical Research Career Guide
Clinical research is a field with great career potential all over the world. Clinical research associates, or CRAs, are one of the most sought-after positions in the field. CRAs monitor labs and research processes. It is a position that offers financial security and many traveling opportunities for work. In this article, we have put together a short work guide on becoming a CRA in Montenegro.
Salary:
The average salary for a Clinical Research Associate (CRA) in Montenegro in 2024, as per data from SalaryExplorer, is 3,090 EUR per month. However, this figure can vary depending on factors such as experience and location.
For those with less than 2 years of experience, the average monthly salary stands at 1,610 EUR. After gaining 2 years of experience, there is a notable increase of 33%, bringing the average salary to 2,150 EUR per month. With 5-10 years of experience, CRAs can expect a further increase of 48%, resulting in an average salary of 3,180 EUR per month.
Overall, salaries for CRAs in Montenegro can range from 1,420 EUR to 4,910 EUR per month, contingent upon individual circumstances.
Requirements to Work:
The minimum education requirement to work as a CRA in Montenegro is a bachelor’s degree. However, investing in a master’s degree will increase your qualifications and earning power. SalaryExplorer has shown that CRAs with a master’s degree have a 93% higher average salary than CRAs with just a bachelor’s degree.
Although clinical research is a global field, job requirements and salaries can differ from place to place. After reading this guide, we hope that you have a better understanding of what it is like to work in Montenegro. If you want to learn more about clinical research and becoming a CRA, please check out our CRA training program and our articles below to learn more about the international job scene.
Explore Courses for Clinical Research Careers in Montenegro
Courses Available:
CRO in Montenegro
Offices in Montenegro:
E-mail: info@clinres-farmacija.hr
Web: http://www.clinres-farmacija.hr
Clinres farmacija,Bul. Svetoga Petra Cetinjskog 114/I,81000 Podgorica,Crna Gora