Argus Software - Oracle Argus Safety Database Training
Because of the continual change in the FDA and International regulations concerning drug safety, there is a need for a more adaptive and comprehensive drug safety software. Argus has a good capacity to mine, report and track safety data. The focus has shifted to a ‘duty of care’ through proactive safety management – rather than just regulatory compliance. CCRPS provides pharmacovigilance training in oracle argus safety database.
Download the Oracle Argus Safety Training PDF here
Oracle Argus Training and Certification
Oracle Argus Safety Certification is a 6 part course that will teach you everything there is to know about the management of drug safety data when using the Oracle Argus Safety application. See a skim through of our Argus Safety Database training here below. Our certification is an argus safety database training online that is internationally accepted for argus safety online training in the United States, Australia, Bangalore, Canada, Europe, Germany, Mumbai, UK and other major clinical trial running countries.
Argus was greek mythical giant with 100 eyes who was assigned by Zeus’ wife (Hera) to keep a vigilant watch of Zeus’s lover (Io) who had been turned into a cow.
Softwares used in Pharmacovigilance
Although Oracle Argus Safety is the most popular tool for safety monitoring, we have also included other tools that are used frequently and you should at least have a basic understanding of them from reading their user manuals (you can google the manuals) if you encounter them. It is worth mentioning that most Fortune 500 companies and CROs prefer the use of Oracle Argus Safety as the standard way of handling drug safety data.
Oracle Argus Safety
ArisG LIFESPHERE® MultiVigilance
System (AERS) i.e. FAERS, VAERS online datasets for safety monitoring and online filing of AEs
arisg safety database training
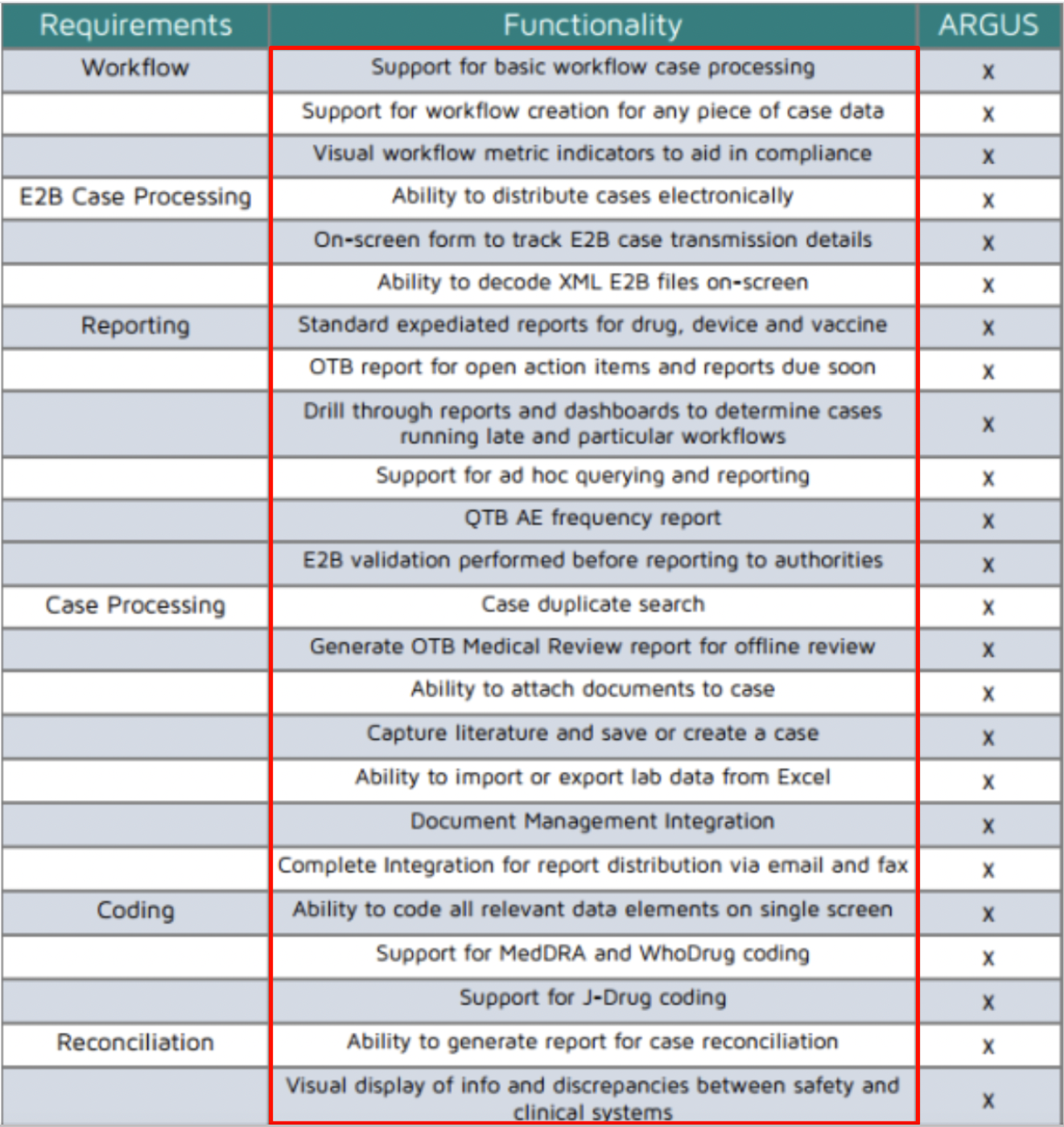
The main essential features of Argus safety database include:
Applicable to all product types: Drugs, devices, vaccines, biologics, and gene therapies.
Fits most business requirements with some ability to customize. Fully Flexible configuration: Best for complex businesses, needing full customization. Semi-Flexible configuration: Allows system to be tailored to your business needs.
Built-in workflow: Migrate cases through workflow tasks.
Complete audit history: 21 CFR Part 11 compliance.
Integrated query module: Simple and complex analysis and surveillance.
Report and submissions tracking: FDA/International expedited and periodic reports.
Document Management: Attach any type of document.
Dictionary Management: MedDRA and WHODrug coding and versioning.
Argus Drug Safety Training Free
Without our certification or CME credits, do you need our certification or CME credits? Before you sign up for our course and spend your hard earned money, why not check out what our students say about our training? If you’re already training in PV/Drug safety, or have already hired, but are just unsure of how to click through all those buttons? If you are trying to establish a more efficient flow in navigating argus drug safety database? Keep reading to find out how to teach yourself for free in the following below!
While certification helps boost the resume, most of us just need to learn what we need to do so we made a free training guide for everyone.
Even without a argus safety certification course, students can trial the application themselves by downloading a free version of argus safety here. For argus safety database software free download see the Oracle® Argus Safety Installation Guide and download version 7.0 instead. FYI: Argus Safety installation requires a database first installed, to our knowledge version 7 is open-source to download so you can host argus safety.
ORACLE Argus User Guide Links
Argus Suite: Argus Safety (Argus Affiliate, Argus Dossier, Argus Interchange, Argus Unblinding), Argus Safety Japan, Argus Insight, Argus Mart, Argus Analytics
Safety One Suite: Safety One Intake
Empirica Suite Empirica Signal and Topics, Empirica Study
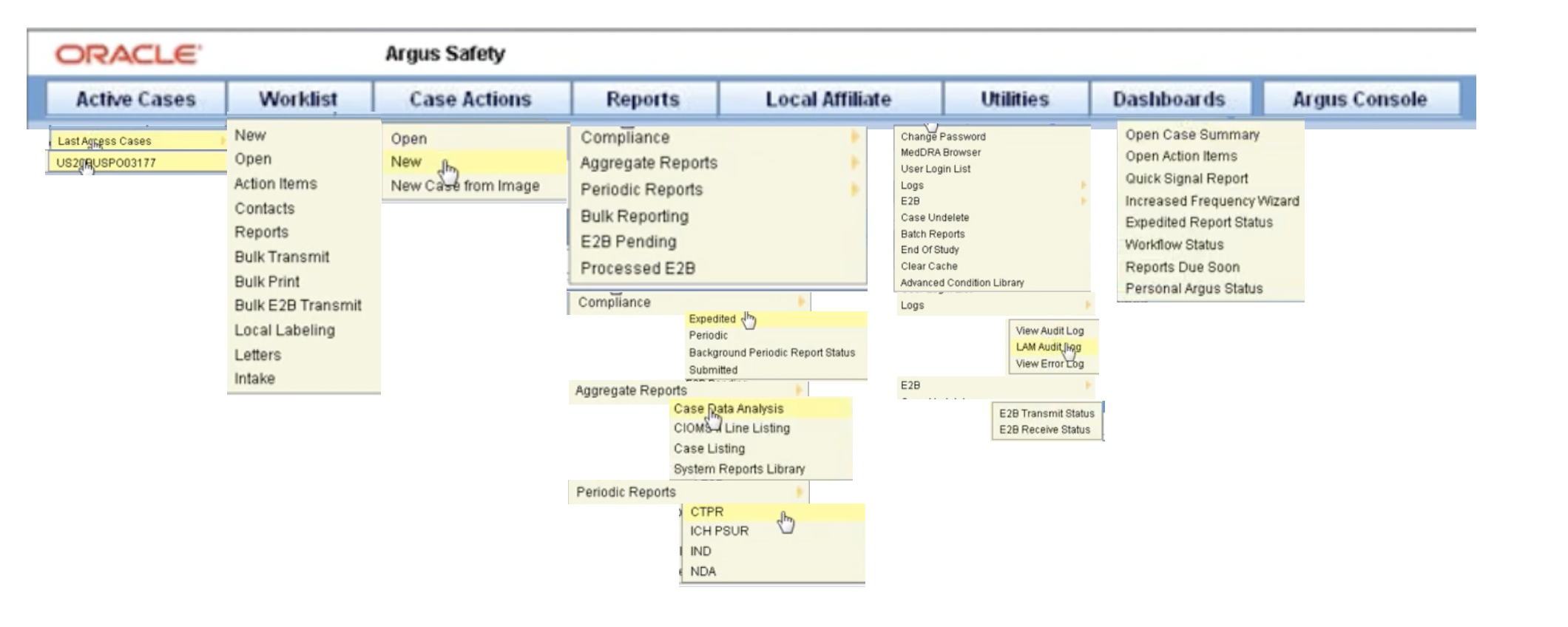
Train yourself in Oracle Argus Safety by clicking on each user guide link here and making sure you understand the steps. Get access to a demo Oracle Argus Safety software here.
Know Your Basics: Log in, Change your password, Begin on the Personal Argus Status page, Use the quick launch toolbar, Use field validations, Acceptable date formats, Use other (than English) language text, Enter reasons for missing (null flavor) data (Video), Log out, Dynamic workflow indicator, Integrate with Argus Insight, Case form user preferences
Enter Case Data: Create a new case (Video), Enter general information (Video), Enter patient information (Video), Enter products information (Video), Enter event information (Video), Add attachments to your case
Process Case Data: Access cases (Video), Process cases, Review cases (Video)
Filter Cases with Advanced Conditions: Create a single filter (Video), Create a set of related filters (Video), Share filters with other users, Modify a filter, Find filters, Use filters to view the case series list (Video), Use advanced condition library to access filter
Code an Adverse Event Term: Autocode a term, Manually code a term
Manage Your Expedited Report Submissions: Schedule an expedited report, Submit your expedited reports, Track your expedited report submissions, Manage your incoming ICSRs
Manage Your Periodic Report Submissions: Prepare content for periodic reports, View scheduled periodic report information, Submit a periodic report, Track your periodic report submission
Work in a Multi-tenant Argus Environment: Access your global worklist items
Configure Argus Settings: Manage access to Argus, Configure code lists, Configure products and licenses, Configure clinical studies, Configure reporting rules
Customize Your Reports Through BIP: Enable BI Publisher aggregate reporting, Configure the Argus BIP integration, Generate and modify a case series, Run a BIP periodic report through Argus Safety, View an aggregate report from Argus Safety, Argus Insight compatibility
Argus safety training videos:
This 4 hour argus safety lecture series we love (watch it, seeing someone go through the software once is more than enough training to start your job.)
Also see this wonderful argus drug safety training video lecture series on Oracle Argus Safety
Conclusion
Oracle Argus Safety is a leading pharmacovigilance software used worldwide for drug safety monitoring and compliance. Whether you seek certification or simply want to navigate the system efficiently, various training options are available. From essential features to hands-on practice with a demo version, learning Argus Safety equips professionals with the skills needed for effective drug safety data management. Start your training today to enhance your expertise in pharmacovigilance.
Explore Courses for Clinical Research Career
Courses Available:
Frequently Asked Questions (FAQs)
-
Oracle Argus Safety is a pharmacovigilance software used for managing drug safety data, adverse event reporting, and regulatory compliance. It helps pharmaceutical companies, CROs, and regulatory agencies track, analyze, and report drug safety information efficiently.
-
While not mandatory, Oracle Argus Safety certification enhances your job prospects in pharmacovigilance. Many pharmaceutical companies prefer professionals with Argus Safety expertise for drug safety and regulatory compliance roles.
-
Some organizations provide free training materials, user guides, and demo software installations. You can also explore Oracle’s official documentation and online forums for self-paced learning.
-
Oracle Argus Safety includes workflow automation, case management, regulatory reporting, audit tracking, MedDRA and WHO Drug coding, and document management. These features ensure compliance with global pharmacovigilance regulations.
-
You can enroll in Oracle-certified training programs or courses offered by pharmacovigilance institutes such as CCRPS. Online platforms also provide Argus Safety certification with flexible learning options.