CCRPS Reviews and Graduate Outcomes
Case Studies, Graduate Placements, & Reviews
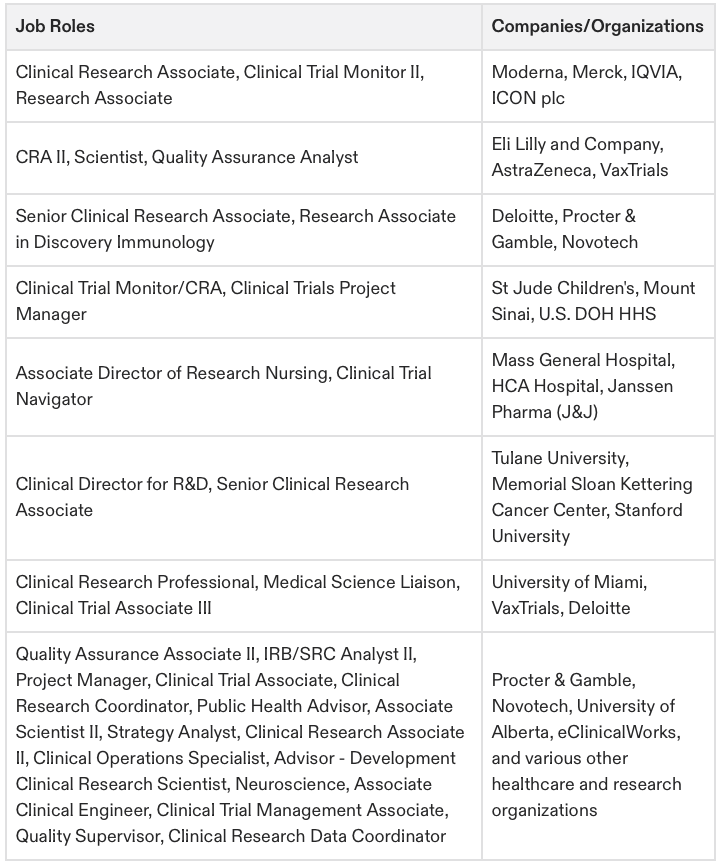
CCRPS Graduate Survey Results
Graduate career data matters more than reviews alone. Keep scrolling to see our compilation from hours of graduate case study interviews and video testimonials as well. Review LinkedIn to see over 2,000 researchers utilizing our badges to optimize career success after course completion. Our training is only as effective as your dedication to the content, but with 100s of videos, interactive lessons, and example-focused applications; we know how to make you truly comprehend the role you are completing. Check out our graduate survey results based on job positions post enrollment date to see where some of our graduates have landed roles. For any further questions about our survey and to review results please email nader@ccrps.org. Click each table to expand results.
Choosing a clinical research certification is not about whether the curriculum sounds impressive or the marketing behind a vague course. It is about whether the training holds up when the job gets real. When you need clean documentation, defensible decisions, and repeatable workflow under timelines you did not control. We focus on training you through applications and examples that make dry guidelines digestible, one of the core reasons our program reach has expanded globally in the past 9 years directly to researchers (not just mandated “job training” modules). That is where most “good” courses reveal their gaps.
The reviews below come from learners across CCRPS tracks, including CRC, CRA, Pharmacovigilance, Project Management, Principal Investigator, Good Clinical Practice, Clinical Trials Assistant, and Medical Liaison. Their feedback consistently points to the same outcome: confusion gets replaced by structure. People do not just say “great course.” They describe what changed, what they can do now, and why the material is something they return to when switching studies or preparing for interviews.
You can review the full program details and course-specific reviews here:
https://app.ccrps.org
View Alumni Testimonials and Graduate Case Study Interviews
CCRPS Reviews and Case Studies
From IMG to Clinical Research Coordinator
Lisa-Pierre shares: “This course not only met but exceeded my expectations with its thorough curriculum and insightful modules.”
From IMG to Clinical Research Coordinator
Unber Mahmood says: “The hands-on activities integrated throughout the course really helped solidify my understanding of complex concepts.”
Promoted to Senior Startup Specialist
Justin Scott Brathwaite shares: “I appreciate how the course was structured—very interactive and engaging from start to finish.”
From Physical Therapist to Clinical Researcher
Celia Moon states: “The in-depth content and expert instructors provided me with invaluable insights into the field.”
ICH GCP Usability Confidence
Stephanie explains: “Thanks to this course, I feel more competent and confident in my role.”
Enjoyed Clinical Research Training through Examples
Marta Marszalek shares: “The real-world examples used throughout the course were incredibly useful for applying theory to practice.”
From Clinical Research Receptionist to Certified Study Coordinator
Katie Decker says: “I highly recommend this course for its comprehensive approach and practical applications.”
From International CRC to U.S. Lead CRC and CRA
Aishwarya Sukumar mentions: “The flexible online format allowed me to balance my studies with my professional commitments seamlessly.”
Learning to Lead Safety Associate
Renata Noronha states: “The course materials were clear, well-organized, and directly applicable to my work.”
From IMG to Securing Multiple Roles
Dr. Vrushali Borawak explains: “Joining this course was a pivotal step in my career advancement.”
From Physician to Confident Drug Safety Specialist
Rabiea Bilal shares: “The course provided a robust foundation in the field, which was critical for my professional development.”
From Plant Biologist to Clinical Recruitment Administrative Coordinator
Olajumoke Owati says: “This program is a gateway to extensive knowledge and skills in a supportive learning environment.”
From International PV Roles to North American Market Success
John Vinil shares: “The detailed modules prepared me excellently for real-world applications.”
From Educational Research to Clinical Trials Project Manager
Rose Hyson states: “I was able to immediately apply what I learned in the course to my job.”
From Masters in Health Safety to Clinical Researcher
Ossai Opene says: “I will say quality of delivery, quality of the materials.”
ICH GCP Made Her More Confident
Aastha Shah explains: “This course just overall did a really good job going in depth, which wasn’t just for the sake of covering content.”
From Grant Program Manager to Leading Clinical Trials at UCSF
Hannah Fischer shares: “It really did a great job of the full scope of clinical research from start to finish. Since completing the course, I've received a promotion at work.”
From Clinical Research Intern to Regulatory Affairs Associate at UPenn
Scott Boyle explains: “I've completed this course. It's helped me get my job in regulatory affairs at a clinical research site.”
From Clinical Research Monitor to Chief Medical Officer for CRO
Maria Lopez states: “And CCRPS has a complete, really good approach to quality and safety because we are all physicians.”
From International Pharmacist to Pharmacovigilance
Ijeoma Osunwa explains: “Certification benefits were gaining clarity on research topics and standing out for clinical research roles.”
CCRPS Reviews
Choosing a clinical research certification is not about whether the curriculum sounds impressive on a landing page. It is about whether the training holds up when the job gets real. When you need clean documentation, defensible decisions, and repeatable workflow under timelines you did not control. That is where most “good” courses reveal their gaps.
The reviews below come from learners across CCRPS tracks, including CRC, CRA, Pharmacovigilance, Project Management, Principal Investigator, Good Clinical Practice, Clinical Trials Assistant, and Medical Liaison. Their feedback consistently points to the same outcome: confusion gets replaced by structure. People do not just say “great course.” They describe what changed, what they can do now, and why the material is something they return to when switching studies or preparing for interviews.
You can review the full program details and course-specific reviews here:
https://app.ccrps.org
What CCRPS graduates keep highlighting
Across these testimonials, several themes show up again and again:
Depth that feels usable, not “more content.”
Learners do not just call the course detailed. They describe it as detailed in a way that improves how they work, not just what they know. That usually shows up as clearer next steps, stronger confidence in role expectations, and fewer “I think this is right” moments.Career alignment that makes resumes and interviews stronger
Multiple reviews signal a direct career impact: better targeting for roles, stronger interview readiness, and the ability to explain experience with real operational language. That is what hiring teams trust. Not motivation. Operational clarity.A reference you actually revisit between trials
A strong training product becomes something you return to. One review specifically mentions going back to the course every time they switch to a new trial to refresh knowledge. That is the opposite of “watch once and forget.” That is workflow support.Reduced intimidation through structure and navigation
Clinical research has a learning curve. Several learners mention they felt intimidated at first, then found the syllabus, instructors, and materials easy to follow. That is not a small detail. Good training makes complex material sequenced and usable.Credibility without overpriced or year-long commitments
What makes these reviews valuable is that they are not vague praise. They mention affordability relative to what people have paid elsewhere, clarity of course design, and outcomes like being better prepared for interviews. That specificity is a credibility signal.
Graduate stories and what each one reveals about the program
CRC: When “detailed” actually means job ready
One learner described their CRC experience in a way that is exactly what most new coordinators need. Not inspiration. Direction. They called it a detailed and excellent course and recommended it for anyone who wants to become a Clinical Trial Coordinator.
That matters because the CRC role is usually where people feel the most pressure early. You are expected to track the moving parts, communicate clearly, and maintain documentation standards while learning a new environment. The review is short, but the signal is strong: the course did not feel abstract. It felt directly applicable.Graduate: Atia Sheereen
Review theme: clear CRC readiness through structured training
CRA: The “stepping stone” that monitors keep using
A CRA learner framed the course as a foundational stepping stone for monitors and then added the detail that matters most. They said it was the best course to take if you want to move up the ladder in clinical trials. They also described it as affordable compared to other training they have paid for, and noted they have paid three times as much elsewhere to learn what is covered in just a chapter.The strongest line is operational: they refer back to the course every time they switch to a new trial to refresh their knowledge. That tells you the content is not just definitions. It is a practical guide that helps you re orient quickly when protocols change and you have to ramp fast.
Graduate: George Grudziak
Review theme: monitoring foundation plus a reusable reference for new trials
Pharmacovigilance: When a course directly fixes “resume mismatch”
One PV learner described a problem that blocks many applicants. They were having poor luck applying to PV jobs because their resume was not targeted toward the field. After taking the course, they added it to their resume and found the knowledge review helped them prepare for interviews better than their previous training.
That is a high value outcome because PV interviews often test practical comprehension. People get filtered out when they cannot explain case processing logic, safety reporting expectations, or how they think about compliance. The learner also mentioned finishing in a short time by watching videos at 2x, which suggests the course is structured enough to remain digestible even when someone moves quickly through it.
Graduate: Mohanad Kour
Review theme: interview readiness and a stronger PV positioning signal
Project Management: When navigation and clarity remove intimidation
A project management learner described something that is surprisingly common. They were originally intimidated by the materials and everything they had to learn. Then they said the syllabus, instructors, and course material were easy to follow and learn from, and they were very happy they completed the course.
That matters because project management in regulated environments is not “generic PM.” You need to understand constraints, stakeholder pressure, and documentation discipline without falling into chaos. A course that reduces intimidation without oversimplifying is usually doing one thing well: it is giving learners a map, not just topics.
Graduate: Natalie Johnson
Review theme: structured learning path that lowers overwhelm and increases completion
Principal Investigator: When the value is direct career usefulness
A PI track learner summarized the impact in a single sentence. They called it a great course and said it was very helpful to their career as a Principal Investigator.
Even short reviews can be meaningful when they match the reality of the role. PIs are judged on oversight, decision making, and the integrity of how they lead studies. A course being “helpful to my career as a PI” signals it gave something practical enough to influence how they operate, not just what they can repeat.
Graduate: Liliana Ruiz Leon
Review theme: career support for PI level responsibility
Good Clinical Practice: When “easy to understand” is not a soft compliment
A GCP learner described the course as effectively designed to be easily understood by candidates and even said the certification examination is designed to be easily accessed.
This matters because GCP content can become either too vague or too heavy. If it is vague, it is not useful. If it is heavy without structure, people retain fragments and fail to apply it. When someone says it was easy to understand, what they often mean is the course translated compliance expectations into clear behavior and clear process, which is exactly what GCP should do.
Graduate: Syeda Sana Sakinatul Kubra
Review theme: accessible structure without losing clarity
Clinical Trials Assistant: When the feedback still includes real nuance
A learner reviewing the Research Assistant style training described it as an excellent course and added a useful nuance. They said some lectures are a bit quick and one has to hear them again.
That kind of feedback is valuable because it reads like a real experience, not marketing language. It also signals there is enough density that replaying sections adds value. For Clinical Trials Assistants and entry level research roles, repetition is not a flaw. It is often how people build confidence with terminology, workflow, and role expectations.
Graduate: Adolfo Enrique Gonzalez Aleman
Review theme: dense, practical learning that benefits from review
Medical Liaison: When audit and inspection knowledge changes how you see the industry
A learner focused on audit and inspection described a specific eye opener. They said it revealed how some investigators and sponsors can influence subjects’ decisions to recruit or retain them in a research study. They also said they learned methods toward compliance, recruitment, and retention of subjects without any altruistic reasons.
That is not a generic compliment. It is a perspective shift. It suggests the course taught them to see clinical research through a sharper lens: ethics, compliance pressure, recruitment tactics, and how decisions can be influenced. For Medical Liaison and medical affairs adjacent professionals, that kind of understanding supports better stakeholder judgment and stronger professional boundaries.
Graduate: Adebowale Adewunmi
Review theme: audit literacy and a clearer view of compliance realities
What these reviews imply about the learner experience
These testimonials do not read like generic praise. They repeatedly point to practical outcomes that map to real clinical research work:
Structure that reduces confusion when shifting studies
Material that supports interview readiness and career positioning
Training that learners revisit because it stays useful
Navigation that reduces intimidation and increases follow through
Compliance oriented thinking that improves professional judgment
In other words, graduates are not only saying “this was good.” They are describing why it made them more capable in concrete terms.
Transparency note about testimonials
CCRPS does not claim that one course guarantees employment, income, promotions, or identical outcomes for every learner. Career outcomes depend on variables such as prior experience, consistency, communication skill, the roles you apply for, local market conditions, and how you present your capability.
Testimonials are shared because they show what learners experienced, not because they function as promises. The credibility signal here is not “everyone will have the same outcome.” The credibility signal is “learners can name what changed and why it mattered.”
Common questions about CCRPS reviews
Are these reviews from real CCRPS learners?
Yes. These testimonials were provided by learners and reflect personal experience. The most trustworthy reviews include specifics like interview preparation, course navigation, affordability comparisons, and returning to the material when switching trials.Why do some reviews emphasize “structure” and “easy to follow”?
Because clinical research is not hard فقط because of the content. It is hard because of the context. Multiple stakeholders, strict documentation expectations, and role ambiguity can overwhelm learners. A structured course turns complexity into a sequence you can execute.Do I need prior experience to benefit from CCRPS training?
Not necessarily. Some learners are early career and want role clarity. Others are already working and want stronger structure, better readiness for new trials, or clearer positioning for interviews. The common thread is not background. It is the desire for defensible competence.Why would an experienced professional still take a CCRPS course?
Because experience does not always equal system. People can work in trials and still feel inconsistent when switching protocols, handling documentation expectations, or preparing for audits. A strong course helps convert experience into repeatable, explainable workflow.What should I look for when comparing clinical research certifications?
Look for specificity and operational outcomes. Do reviews mention returning to the course, interview readiness, reduced intimidation, or concrete improvements in how they work. Vague praise often signals motivation. Specific details signal learning transfer.Where can I see exactly what is included in the program?
CCRPS keeps the syllabus accessible so learners can evaluate the scope and structure directly. You can review the full program details here:
https://app.ccrps.org
Ready to review the program behind these testimonials?
If you want the fastest way to evaluate CCRPS, do not start with marketing language or vague programs. Start with reviewing and comparing the curriculum. Review the syllabus, compare the scope to your role goals, and decide whether the structure matches the level of professionalism you want your work to reflect.
You can review the full program details here:
https://app.ccrps.org