Clinical Research Certification
CCRPS Provides Accredited Online Clinical Research Training To Start Or Grow Your Career in 1 to 8 Weeks. Access 2025 Course Updates with 600+ New Interactive Lessons. Call +1 (239) 329-9837 or chat 24/7 advisors below.
“Joining this course was a pivotal step in my career advancement.” – Dr. Vrushali Borawak, From CRC to CRA to Project Manager with CCRPS
Trusted with 8 Years of Graduate Success and Thousands of Graduates Working Around The Globe.
Get Instructor-led Weekly Live Review Sessions, Mentorship, and Job Support.
Join our free 26,000 member community as we expand with free training and educational resources this year.
Graduate Success Stories
1. From IMG to Clinical Research Coordinator at Columbia University: “This course not only met but exceeded my expectations with its thorough curriculum and insightful modules.” -Lisa-Pierre (view full case study)
2. From IMG to Clinical Research Coordinator “The hands-on activities integrated throughout the course really helped solidify my understanding of complex concepts.” -Unber Mahmood (case study summary)
3. Promoted to Senior Startup Specialist in Clinical Trials: “I appreciate how the course was structured—very interactive and engaging from start to finish.” -Justin Scott Brathwaite (transcript summary)
4. From Physical Therapist to Clinical Researcher: “The in-depth content and expert instructors provided me with invaluable insights into the field.” - Celia Moon (case study summary)
5. ICH GCP Confidence: “Thanks to this course, I feel more competent and confident in my role.” - Stephanie (case study summary)
6. Enjoyed Clinical Research Training through Examples “The real-world examples used throughout the course were incredibly useful for applying theory to practice.” -Marta Marszalek (view full case study)
7. From Clinical Research Receptionist to Certified Study Coordinator with CCRPS: “I highly recommend this course for its comprehensive approach and practical applications.” - Katie Decker(view full case study)
8. From International CRC to U.S. Lead CRC and CRA: “The flexible online format allowed me to balance my studies with my professional commitments seamlessly.” - Aishwarya Sukumar (view full case study)
9. Learning to Lead Safety Associate: "The course materials were clear, well-organized, and directly applicable to my work.” - Renata Noronha (view full case study)
10. From IMG to securing roles as a CRC, CRA, and now a project manager: “Joining this course was a pivotal step in my career advancement.” - Dr. Vrushali Borawak (view full case study)
11. From Physician to Confident Drug Safety Specialist: “The course provided a robust foundation in the field, which was critical for my professional development.” -Rabiea Bilal (view full case study, now also one of our PV instructors)
12. From plant biologist to clinical recruitment administrative coordinator: “This program is a gateway to extensive knowledge and skills in a supportive learning environment.” -Olajumoke Owati (view full case study)
13. From International PV Roles To North American Market Success: “The detailed modules prepared me excellently for real-world applications.” - John Vinil (view full case study, now also one of our PV instructors)
14. From Educational Research to Clinical Trials Project Manager: “I was able to immediately apply what I learned in the course to my job.” - Rose Hyson (view full case study)
15. From Masters in Health Safety to Clinical Researcher: " I will say quality of delivery, quality of the materials. - Ossai Opene (view full case study)
16. ICH GCP made her more confident in research: "this course just overall did a really good job going in depth, which I feel like wasn't just, it wasn't just covered just for the sake of covering content" - Aastha Shah (view full transcript)
17. From Grant Program Manager to Leading Clinical Trials at UCSF: "it really did a great job of the full scope of clinical research from start to finish. Since completing the course, I've received a promotion at work." -Hannah Fischer (view HF clinical research training case study)
18. From Clinical Research Intern to Regulatory Affairs Associate at UPenn: "I would say since then. I've completed this course. It's helped me get my job in regulatory affairs at a clinical research site." - Scott Boyle (view SB full case study)
19. From Physician to Chief Medical Officer for CRO: "And CCRPS has a a complete, you have a really, really good approach to that. Because that is what we offer to our sponsors, quality and safety, because we are all physicians." - Maria Lopez (view case study)
Email us to review over 15 hours of graduate case study videos or request access to graduate placement data near your location. Start advancing your career today
Why Choose CCRPS?
With thousands of successful graduate placements worldwide, our proven curriculum has continuously been reported by graduates to boost job interview rates, enhance professional knowledge, and secure industry recognition. CCRPS programs are utilized by students from 1,200+ organizations, six government agencies, 308 universities, and result in graduates working at over 1,600 companies (view grad placement/case studies). .
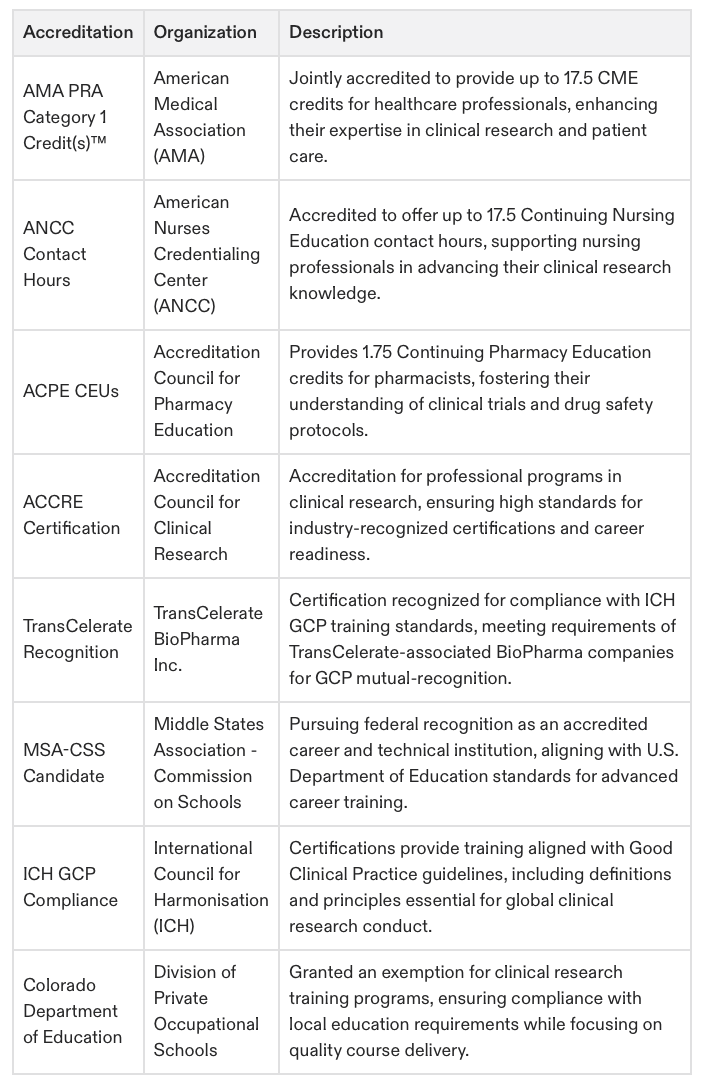
Accredited by ACCRE, Joint Accreditation for CME (ACPE, ANCC, and AMA), partnered accreditation with NHA. GCP recognition by TransCelerate Biopharma. Exemption for vocational training with Colorado Department of Education. Candidate for federal career institution by MSA (view accreditation).
Join our free clinical research community of 26,000 or enroll today with 24/7 course support, 14-day refund policy, and flexible payment plans.
Popular Clinical Research Courses
Lifetime Access to Updated and Expanded for 2025 with Bite-Sized Advanced Modules, Instructor-Led Sessions, and Mentorship.
✔ Good Clinical Practice Certification 70 Modules - 1 Week
Required every 2 years for clinical researchers. Recognized by Transcelerate Biopharma. Updated for E6 R3. Complete 10 modules a day and master good clinical practice guidelines. Interactive advanced modules with videos, review tables, application assessments, weekly live exam review webinar, and 25Q final exam. Get mentorship.
✔ Clinical Research Coordinator Certification 112 Modules - 4 Weeks
Start your clinical research career with comprehensive CRC training. Accredited by ACCRE and for CME ANCC. Finish 28 modules a week and master clinical research coordinator foundations along with senior-level skills, learn specialties, medical devices, multinational trials, and more. 50Q final exam. Get 1-1 mentorship and career support.
✔ Clinical Research Associate Certification 288 Modules (4 Weeks)
Advance your clinical research career with cutting-edge CRA training. Accredited by ACCRE and CME ACPE, ANCC, AMA. Finish 10 modules a day and master clinical research associate foundations along with senior monitoring skills in dozens of specialties, trial types, and niches. Get 1-1 mentorship and career support.
✔ Pharmacovigilance and Regulatory Affairs Certification (4 Weeks, 169 Modules)
Transition as a researcher into pharmacovigilance and regulatory affairs with multi-national advanced training. Joint Accreditation for CME with ACPE. Finish 6 modules a day and master pharmacovigilance and regulatory affairs skills with videos, weekly instructor-led live exam reviews, applications assessments, videos. Get 1-1 mentorship and career support.
✔ Clinical Project Manager Certification (4 Weeks, 155 Modules)
Transition from your research role into management with expert-level skills training. Joint Accreditation for CME with AMA, ANCC, ACPE. Finish 5 modules a day and master advanced project management and clinical research skills. Get 1-1 mentorship and career support.
✔ Medical Monitor Certification (4 Weeks, 133+ Modules)
Work as a clinical research physician monitoring trials with multi-specialty training. Joint Accreditation for CME by AMA/ACME. Finish 5 modules a day and master advanced medical monitoring skills in every specialty with instructor-led live exam reviews and 1-1 mentorship and career support.
✔ Research Assistant Certification (4 Weeks, 114 Modules)
Begin your career in clinical research and gain research experience with cutting-edge training. Accreditation by ACCRE and CME. Finish 4 modules a day and master research assistance in multiple trial designs, protocol skills, and finish quickly with instructor-led live exam reviews and 1-1 mentorship.
✔ Principal Investigator Certification (4 Weeks, 178 Modules)
Enhance sponsor selection and conduct clinical trials with training in master-level skills and all trial specialties. Joint Accreditation by AMA/ACME. Finish 6 modules a day (skim ones you don’t need) and finish in 4 weeks with instructor-led live exam reviews. Obtain mentorship if transitioning into PI roles.
Benefits of CCRPS
Designed for science graduates, researchers, and CROs, our programs deliver advanced expertise to elevate careers and streamline clinical trials staff training (take career quiz).
CCRPS offers advanced clinical research career training for all levels with self-paced clinical research certification for good clinical practice and clinical research job training for roles including research assistant, clinical research coordinator, clinical research associate, pharmacovigilance and regulatory affairs, clinical project management, medical monitor, and principal investigator (view courses).
Enroll today for lifetime access with 2025 updates including over 600+ updated and new interactive lessons (master foundations, learn expert-skills, specialty trials, decentralized trials, and more) with on-demand videos, weekly live review webinars, and bite-sized advanced modules with applications, examples, practicals, review tables, and practice questions.
Accelerate your career with 1-1 mentorship and job support. Access modules 24/7 on mobile or years later with lifetime access.
Clinical Research Courses
Accredited Online Clinical Research Certification Program
Master Good Clinical Practice Certification and Stand Out
70 Advanced Modules I All Researchers I Master The Foundations
ICH GCP Expert: "Thanks to this course, I feel more competent and confident in my role." - Stephanie
-
Good Clinical Practice ensures ethical, safe, and regulatory-compliant clinical trials. Advanced certification validates and expands your expertise, boosting your career and positioning you for advancement in clinical research.
Get Certified Today. -
Earn this comprehensive 7-day, 70-module certification covering topics like informed consent, trial management, and regulatory roles. Flexible, self-paced learning with live webinars with proven graduate success in placements and promotions.
Who It’s For: All clinical professionals and researchers. Take every 2 years (free lifetime access).
Duration: 1 Week.
Career Outcomes: Clinical Research Assistant, Coordinator, Associate, or Regulatory Advisor.
Enroll Now to advance your career.
-
Master guidelines in 7 days with on-demand ICH GCP certification
-
The upcoming ICH E6(R3) focuses on remote monitoring, global harmonization, and updated methodologies. CCRPS is expanding 3x in 2025 to meet these updates. Join the most up-to-date certification today.
Stay Ahead and Enroll. -
Gain advanced knowledge with our course, including strategies for adverse event reporting, global consent ethics, regulatory readiness, and real-world evidence use—skills that set you apart.
Discover More. -
Our Graduates Hired As: Research Assistant, Lab Assistant, Research Coordinator, Research Scholar, Postdoctoral Researcher, Graduate Research Assistant, etc. Intern roles: Research Assistant Intern, Outpatient Pharmacy Intern, Clinical roles: Clinical Affairs Intern, Clinical Fellow, Clinical Nurse, Clinical Operations Manager, Clinical Research Professional Teaching roles: Assistant Professor, Lecturer, Graduate Teaching Assistant, etc. Management roles: Clinical Research Manager, Pharmacy Operations Manager, Associate Director of Clinical Development, Vice President of Clinical Development Specialized roles: Drug Safety Associate, Regulatory Specialist, Scientific Consultant, Medical Laboratory Scientist, Pharmacovigilance Associate Other roles: NHS Primary Care QI Facilitator, Government Healthcare Recruiter, Research Ethics Coordinator (based on review of new job positions after enrollment in course)
Advance your clinical research expertise with the ICH GCP Certification, a must-have credential for professionals in the field. This top-tier Good Clinical Practice Certification provides you with the comprehensive knowledge and GCP trainingneeded to ensure ethical research, regulatory compliance, and data integrity. Ideal for newcomers, healthcare professionals, or industry veterans, this flexible Good Clinical Practice GCP Training sets you apart in a competitive market.
Complete the 50-module, self-paced GCP course in less than a week and earn a globally recognized Good Clinical Practice Certificate. Starting at just $99 with packages as low as $10/month, the program also includes options for live sessions and one-on-one mentorship upgrades to support your success. A 14-day money-back guarantee ensures a risk-free investment.
Whether you're pursuing roles in research, teaching, or management, this prestigious ICH GCP certification positions you for advancement. Graduates secure diverse positions such as Clinical Research Associate, Regulatory Specialist, or Clinical Trial Coordinator, earning salaries from $47k-$78k annually while enhancing their career options.
Explore our advanced GCP courses and achieve compliance with international research standards. This good clinical practices certification equips you with enduring career value in clinical research. Enroll now to earn your GCP certificate and access exciting opportunities in this dynamic field.
Clinical Research Coordinator Certification
116 Advanced Modules I 2 Year Degree I Proven Graduate Success
“The hands-on activities integrated throughout the course really helped solidify my understanding of complex concepts.” -Umber Mahmood
-
A Clinical Research Coordinator (CRC) oversees clinical trials, ensuring regulatory compliance, ethical management, and accurate data collection. Being a certified CRC with our advanced curriculum validates your expertise and enhances working knowledge to help you get noticed, interview better, and be promoted faster.
Who It’s For: Healthcare professionals or graduates with 2-year science degrees.
Duration: 4 weeks.
Career Outcomes: Clinical Research Coordinator, earning $59,000–$80,000.
Learn More.
-
Our 112-hour accredited program is tailored for flexibility to finish in 3 weeks with self-paced online modules and live webinars.
Choose from three pricing tiers to fit your needs:
$399 for core certification.
$999 for live online sessions and job placement.
$1,499 for one-on-one mentorship and full career support.
Complete the course in just 2–4 weeks and step into your new career.
Explore Pricing and Enroll.
-
We provide tools and resources to help you succeed, including:
CRC Guidelines and Compliance Handbooks
FDA Standards and Training Resources
CRC Toolkit
Exclusive Access to Advanced CRC Techniques
Enhance your learning experience and get equipped for your role.
Access These Resources.
-
Stay ahead with insights on decentralized clinical trials, enhanced patient engagement strategies, and advancements in remote trial management. Keep your training up-to-date with lifetime access to our program, which is aligned with industry trends and practices.
Stay Current and Enroll. -
Develop in-demand skills like regulatory compliance auditing, data management, and project leadership. Gain specialized training in areas such as oncology, rare diseases, and regulatory affairs to stand out in competitive job markets.
Learn More About Our Advanced Training. -
Graduates Hired As: Clinical Research Coordinator, Clinical Research Coordinator II, Lead Clinical Research Coordinator, Senior Clinical Research Coordinator, Oncology Research Coordinator, Clinical Study Coordinator, Clinical Research Data Coordinator, Clinical Research Nurse, Clinical Director/ Office Manager, Regulatory Contact/ Clinical Research Coordinator, Clinical Research/Regulatory Coordinator, Clinical Trials Specialist, Research Regulatory Specialist, Certified Clinical Research Coordinator, Clinical Research Specialist, Sr. Director of Clinical Operations
Elevate your career with the Clinical Research Coordinator Certification, an advanced certified clinical research coordinator certification designed for success in clinical research roles. Perfect for career starters, healthcare professionals, or those transitioning fields, this comprehensive clinical research coordinator course delivers the skills, knowledge, and credentials needed to thrive.
Complete this globally recognized crc certification in just 4 weeks with 116 hours of curated content, earning 17.5 CME credits. Gain expertise in essential areas like regulatory compliance, patient recruitment, GCP adherence, data collection, trial budgeting, FDA-regulated studies, and site monitoring through self-paced learning, live webinars, and mentorship.
Starting at $399 with payment plans from $32/month, this clinical research coordinator training offers flexible options and a risk-free, 14-day money-back guarantee.
Graduates of this clinical research coordinator certification online secure roles like Clinical Research Coordinator, Clinical Trials Specialist, and Clinical Study Coordinator, earning $59k-$80k annually while progressing to positions like Clinical Director. Explore opportunities through the crc course catalog and master crc training.
Start your path to excellence with this top-tier research coordinator certification. Enroll today to unlock a dynamic career in clinical research
Start with the Most Advanced Research Assistant Training
150 Advanced Modules I HS Diploma I Accelerate Your Career
"it really did a great job of the full scope of clinical research from start to finish." -Hannah Fischer
-
Research Assistants play an essential role in clinical research, assisting in patient recruitment, managing data collection, and ensuring all regulatory protocols are met. With opportunities to earn $25k–$70k+ annually, achieving certification puts you on the fast track to a rewarding career in healthcare and research.
Learn More About the Role. -
Our 150-hour accredited program includes on-demand video tutorials, live online sessions, and personalized 1-on-1 mentoring. Pricing tiers designed to suit your goals:
$299 – Core certification and exam.
$599 – Live sessions + job placement support.
$999 – Full mentorship + advanced job support.
Complete the program in 3 weeks to begin your career quickly and effectively.
Explore Pricing and Register.
-
Gain access to essential materials and guides tailored for success in clinical research:
Patient Safety & Ethics Protocols.
Data Management and Adverse Event Reporting frameworks.
Tools for Trial Documentation & Regulatory Oversight.
These resources ensure a seamless transition from theory to real-world application.
Access Resources Now.
-
Our cutting-edge curriculum keeps you ahead in the evolving world of clinical trials. Learn about new trial methodologies, remote patient engagement software, and next-gen data collection tools. Graduates are fully prepared to adapt to these advancements.
Enroll Today to Stay Current. -
Master essential skills like:
Executing informed consent processes.
Coordinating trial logistics across multiple locations.
Ensuring compliance with ethical and regulatory standards.
These proficiencies prepare you for lucrative positions with pathways to senior roles.
Build Your Expertise Here.
-
Graduates Hired As: Clinical research assistant, clinical trial assistant, clinical researcher professional, clinical research coordinator, trial assistant, research assistant.
Jumpstart your career in clinical research with the Clinical Trials Assistant Certification, an all-encompassing clinical research assistant training designed for aspiring professionals. This research assistant program equips you with vital skills in clinical trial support, including regulatory compliance, patient safety, trial design, data management, and adverse event reporting.
Complete the most advanced 3-week, 100-module clinical research assistant certification at your own pace and stand out in the job market. With flexible learning options, live sessions, 1-on-1 mentoring, and affordable packages starting at $299 (payment plans from $25/month), this research assistant course is tailored to fit your career goals. Enroll risk-free with our 14-day money-back guarantee.
After completing this program, graduates secure competitive research assistant jobsas Clinical Trial Assistants, Research Assistants, or Regulatory Affairs Assistants. The program prepares you for success in roles with salaries ranging from $25k–$70k+, while opening doors to advancement into higher-level positions with experience.
Whether you're curious about the clinical research assistant salary or eager to grow as a clinical research assistant, this certification elevates your career prospects in a dynamic, high-growth industry. Enroll now and make your mark in clinical research today.
Get Cutting-Edge Clinical Research Associate Certification
165 Advanced Modules I BS Degree I Industry-Recognized
"The in-depth content and expert instructors provided me with invaluable insights into the field." - Celia Moon
-
Clinical Research Associates (CRAs) are essential for ensuring the success of clinical trials, playing a critical role in compliance, quality control, and trial efficiency. They bridge connections between sponsors, investigators, and trial sites, driving advancements in medical research. With CRA certification, you’ll position yourself for high-demand roles earning $60k–$103k annually, with senior roles reaching $120k+.
Discover Your Career Path as a CRA. -
Our 4-week, 250 module program covers more than everything you need to excel as a CRA. Learn at your own pace with on-demand videos, live webinars, and mentorship. Choose the package that fits your goals:
$499 – Self-paced course with certification exam.
$1,299 – Includes live sessions and job support.
$1,999 – Full mentoring and personalized job placement assistance.
Flexible payment options via Affirm and Klarna make this investment in your future accessible.
Start Your Journey Today.
-
Gain access to exclusive tools and materials designed for real-world success:
Comprehensive guides on FDA/ICH GCP regulations.
Monitoring templates for site visits and compliance checks.
Training in remote and digital monitoring tools.
Our training provides what books, guidelines, and other training programs alone cannot to ensure you walk into your first or next role fully prepared and confident.
Access Career-Boosting Resources Now.
-
Elevate your career by mastering the in-depth, expert-level knowledge provided in our comprehensive syllabus. Each module is designed to prepare you for the complexities of clinical research.
Detailed Monitoring Training – Learn advanced techniques for site qualification, initiation, routine visits, and close-out monitoring.
Regulatory Excellence – Gain expertise in adhering to FDA regulations, ICH GCP guidelines, and compliance reporting.
Specialized Tools – Familiarize yourself with cutting-edge platforms like CTMS, EDC, and remote monitoring systems to enhance site management and efficiency.
Diverse Lesson Types – Access a mix of interactive video lessons, PDF guides, quizzes, and real-life case studies to apply your knowledge in practical settings.
This curriculum ensures you’re ready to handle the operational and regulatory aspects that top employers demand, preparing you for an impactful and long-lasting career in clinical research.
Enroll Now to access unparalleled training and expert-driven resources for your success! -
Our program prepares you for the future of clinical research with training in:
Centralized and remote monitoring techniques.
The latest technologies in trial data collection and patient engagement.
Ethical considerations for vulnerable populations.
Stay competitive and meet the expectations of top employers with cutting-edge expertise.
Stay Competitive, Enroll Today.
-
Our recent graduates landed roles including: Clinical Research Associate, Clinical Trial Monitor II, Research Associate, CRA II, Scientist, Quality Assurance Analyst, Senior Clinical Research Associate, Research Associate in Discovery Immunology, Clinical Trial Monitor/CRA, Clinical Trials Project Manager, Associate Director of Research Nursing, Clinical Trial Navigator, Clinical Director for R&D, Senior Clinical Research Associate, Clinical Research Professional, Medical Science Liaison, Clinical Trial Associate III, Quality Assurance Associate II, IRB/SRC Analyst II, Project Manager, Clinical Trial Associate, Clinical Research Coordinator, Public Health Advisor, Associate Scientist II, Strategy Analyst, Clinical Research Associate II, Clinical Operations Specialist, Advisor - Development Clinical Research Scientist, Neuroscience, Associate Clinical Engineer, Clinical Trial Management Associate, Quality Supervisor, Clinical Research Data Coordinator (based on review of new job positions on Linkedin post-enrollment date).
The Clinical Research Associate Certification is designed for professionals seeking career advancement in clinical research. Complete a 250-module clinical research associate training program in just 4 weeks, earning 17.5 CME credits and securing a verified clinical research associate certificate.
This comprehensive program builds expertise in clinical research certification, CRA certification, CRA clinical research associate certification, and clinical trial associate certification. It covers clinical research associate training, CRA training program, and monitoring in clinical trials, medical devices, and biotechnology. Flexible pricing starts at $499, with plans from $42/month.
Graduates secure roles like Clinical Research Associate, Trial Manager, and Medical Device Specialist. Salaries range from $60k–$103k, with potential for six figures. Trusted worldwide, the clinical research associate program equips you to excel globally. Enroll now.
Industry-Recognized Pharmacovigilance Certification
150 Advanced Modules I PharmD/Prior Trials Exp I Leading Program
"I would say since then. I've completed this course. It's helped me get my job in regulatory affairs." - Scott Boyle
-
Pharmacovigilance professionals play a critical role in ensuring drug safety. With this certification, you’ll develop expert skills to identify, assess, and mitigate risks associated with medications, ensuring patient safety worldwide. Certified professionals secure prestigious roles with salaries ranging from $59k to $140k annually, making this an essential step in advancing your healthcare or pharmaceutical career. Package options include:
$499 - Full Certification Course (ideal for self-paced learners).
$999 - Certification + Live Webinars + Job Support.
$1,499 - All-Inclusive Package with One-on-One Mentoring and Complete Job Assistance.
Advance Your Career in Pharmacovigilance.
-
Our expertly designed syllabus prepares you for the complexities of modern drug safety. Key topics include:
Global and regional regulations from FDA, EMA, and WHO.
Adverse event reporting techniques, including ICSRs and PSURs.
The complete signal management lifecycleinvolving detection, validation, and action prioritization.
Specialized skills in vaccine pharmacovigilanceand managing post-authorization surveillance activities.
Hands-on training with industry-standard tools like the Argus Safety Database and MedDRA coding.
Risk assessment strategies and real-world case studies for practical application.
This rounded curriculum ensures you gain critical skills to secure roles like Pharmacovigilance Manager, Drug Safety Specialist, and more.
Explore the Full Syllabus and Advance Your Career. -
Gain insights into global and regional regulations, including FDA, EMA, and WHO guidelines and dozens of other countries. Learn how to manage compliance across diverse healthcare markets while maintaining the highest safety standards. This certification positions you as a globally recognized expert in drug safety and regulatory affairs. Packages are designed for every learner's needs.
Enroll Now to Gain Regulatory Expertise. -
From identifying adverse events to crafting risk mitigation strategies, this program equips you with real-world skills to excel. Plus, with live webinars, 1-on-1 mentorship, and dedicated job placement support, you’ll confidently take on roles like Pharmacovigilance Manager, Drug Safety Specialist, or Senior Analyst. Pick from three pricing tiers to maximize your learning potential.
Join the Leaders in Pharmacovigilance. -
Pharmacovigilance is evolving, and so is our certification. The 2025 updates to the curriculum include:
Integration of AI and machine learning tools for enhanced adverse event detection and signal prioritization.
Comprehensive training on new post-market surveillance guidelines from global health authorities, including updated FDA and EMA standards.
Advanced techniques for monitoring safety in biologics and gene therapies, ensuring you’re equipped for the future of pharmacology.
New modules on emerging topics like real-world evidence (RWE) and digital pharmacovigilance strategies.
Access to the latest versions of Argus Safety Database and MedDRA coding applications, ensuring you train with up-to-date industry tools.
These updates empower you to lead in a rapidly transforming field, giving you a competitive edge to secure top industry roles.
Enroll Today to Access 2025 Updates and future-proof your career -
Recent graduates of the program have secured an impressive range of roles across the pharmacovigilance, drug safety, and regulatory affairs sectors. These positions include Regulatory Affairs Associates, Pharmacovigilance Scientists, Senior Managers in Medical Safety and Quality, Product Safety Managers, Clinical Pharmacists, and even Vice Presidents of Operations and Clinical Development. They are thriving as leaders in roles such as QPPVs, Epidemiologists, Safety Analysts, and Directors of Pharmacovigilance Departments. The diversity spans from entry-level analysts to senior-level executives in esteemed fields, showcasing the program's ability to open doors to lucrative and high-impact careers worldwide.
The Pharmacovigilance Certification Course prepares professionals for careers in drug safety certification, pharmacovigilance certification, and regulatory affairs certification. Learn through an accredited online pharmacovigilance training with over 150 topics covering pharmacovigilance courses, regulatory affairs courses, and quality assurance.
Acquire a globally recognized pharmacovigilance certificate course ideal for roles in drug safety pharmacovigilance certification or certificate in regulatory affairs. Designed for flexibility, complete the program at your own pace starting at $499, with payment plans from $42/month.
Graduates secure roles like Pharmacovigilance Scientist, Product Safety Manager, and Regulatory Affairs Associate with salaries from $70K–$120K, often exceeding six figures. Trusted worldwide, this pharmacovigilance course in the USA is your key to success. Enroll now.
Learning to Lead Safety Associate: "The course materials were clear, well-organized, and directly applicable to my work." - Renata Noronha
Advance Your Research Career with Clinical Project Manager Certification
150 Advanced Modules I Prior Trials or PM Exp I Leading Program
"This program is a gateway to extensive knowledge and skills in a supportive learning environment." -Olajumoke Owati
-
Clinical Research Project Managers are essential in the planning, execution, and success of clinical trials, ensuring projects meet regulatory standards, stay on time, and within budget. This certification equips you with the specialized knowledge and skills required to excel in high-demand roles with salaries ranging from $80k to $160k annually, positioning you for leadership opportunities in one of the fastest-growing healthcare fields.
Whether you're transitioning from a clinical research associate role or building upon existing project management skills, this course provides a step-by-step roadmap to achieve professional success. Pricing packages offer flexibility for all learners:
$499 - Course + Certification Exam.
$999 - Course + Live Sessions + Job Support.
$1,999 - Comprehensive Package with 1-on-1 Mentoring and All Job Placement Features.
Start Your Training and Secure Your Future.
-
Kickstart or advance your career with a curriculum designed by industry experts. This program offers over 150, providing you with the tools needed to excel in clinical trial project management. Highlights of the syllabus include:
ICH GCP Essentials:
Gain a thorough understanding of global regulations and ethical guidelines like CFR 21 Part 11, research ethics, and specialized protocols for vulnerable populations (children, pregnant women, and mentally incapacitated individuals).Fundamentals of Clinical Project Management:
Learn how to create project plans, manage budgets, communicate with stakeholders, and coordinate multi-center trials to ensure seamless trial execution.Roles and Relationships in Clinical Trials:
Understand the responsibilities of key players like sponsors, CROs, IRBs, investigators, and DSMBs, while learning how to navigate collaborative networks successfully.Advanced Clinical Trial Design & Methodology:
Delve into the mechanics of trial phases (0-4), adaptive trial designs, protocol creation, inclusion/exclusion criteria, and strategies for randomized controlled trials.Problem Solving and Risk Management:
Develop the ability to preemptively identify, assess, and mitigate risks, focusing on consistent project delivery even under challenging circumstances.
This comprehensive training delivers both foundational knowledge and advanced expertise tailored to real-world applications in clinical research project management.
Explore the Full Syllabus and Begin Your Journey Today. -
Graduates of this certification have successfully transitioned into roles such as Clinical Trial Project Manager, Program Manager, Research Nurse Manager, and Data Safety Monitoring Specialist. Beyond classroom success, we prioritize launching your new career through dedicated support:
1-on-1 Mentorship: Receive personalized guidance from industry veterans to refine your career goals.
Job Placement Assistance: From resume crafting to interview prep, we help you stand out. Access direct connections within an expansive clinical research network.
Success-oriented services include post-graduation access to updates and career enhancement resources.
-
Stay competitive by learning cutting-edge practices like adaptive clinical trial designs, advanced risk-based monitoring techniques, and the integration of decentralized trials. The program also explores the latest tools for patient engagement and data analytics, ensuring you’re prepared to lead in an innovative healthcare environment.
Stay Competitive and Enroll Today. -
Learn the specialized competencies employers demand, including:
Creating comprehensive Project Management Plans.
Coordinating multi-site trials and ensuring smooth communication with CROs, sponsors, and investigators.
Managing regulatory reporting requirements for sophisticated trial models.
This training positions you for senior roles like Clinical Trial Manager, Program Director, or Global Project Leader, with potential earnings of $160k+ annually.
Advance Your Career With Confidence.
-
Our recent graduates landed roles including: Clinical Trial Project Manager, Research Nurse Manager, Clinical Research Coordinator-Data Manager, Clinical Research Associate, Transdisciplinary Research Project Manager, IT Project Manager in Clinical Research, Publicly Funded Research Project Manager, etc.
Advance your career with the Clinical Project Manager Certification, the ultimate program designed to equip professionals with the expertise needed for success in clinical project management. Whether you’re exploring what training is required to be a clinical project manager or preparing to thrive as a clinical trial project manager, this certification is your gateway to leadership roles in pharmaceuticals, biotech, and medical devices.
Build in-demand skills in clinical research project management, project management in clinical research, and project management clinical studies. Master essential tools for resource allocation, risk assessment, data monitoring, and stakeholder communication. Land top clinical project manager jobs with salaries ranging from $80K–$160K+.
Self-paced course completed in just 4 weeks, with optional live sessions. Includes mentorship, live support, and interactive study tools. Earn 17.5 CME credits and an industry-recognized clinical project manager certification. Flexible payment plans starting at $42/month with a 14-day money-back guarantee.
Graduates of this program secure prestigious roles such as clinical study manager, clinical project manager, clinical trial project manager, or clinical research project manager and utilize tools like the best free clinical project manager protocol checklists to excel in their field.
If you’re ready to boost your earning potential with a proven clinical project manager salary and take the lead in clinical trial project management, enroll today. This clinical project management certification is all you need to thrive in the competitive and rewarding field of clinical research.
Improve Trial Outcomes with Principal Investigator Certification
165 Advanced Modules I Active MD or PI /SubI I Trusted by Groups
"I appreciate how the course was structured—very interactive and engaging from start to finish." -Justin Scott Brathwaite
-
Principal Investigators (PIs) hold a pivotal role in clinical research, ensuring trials are conducted efficiently, ethically, and in compliance with regulatory standards. PIs are responsible for designing protocols, managing multidisciplinary teams, and safeguarding participant safety. Achieving certification as an Advanced Principal Investigator helps physicians gain in-depth expertise, making them more competitive for roles with salaries ranging from $42k–$279k annually. Gain advanced knowledge to excel in oncology, cardiology, neurology, and many other specialties.
Explore Career Opportunities as a PI. -
The Advanced Principal Investigator Physician Certification (APIPC) stands as the gold standard in PI training. This flexible program, offering 17.5 CME credits, is designed to be completed in as little as 1–3 weeks, at your own pace. Gain expertise in:
Clinical Trial Design – From Phase 0 to Decentralized Studies.
Regulatory Compliance – Master FDA guidelines, IRB processes, and global ethics.
Leadership and Collaboration – Build and manage cross-functional teams for seamless research execution.
Flexible tuition options include:$499 – Course with certification exam.
$999 – Includes live sessions and job placement support.
$1,499 – 1-1 mentorship combined with career guidance for PIs.
View Packages and Enroll Today to advance your career.
-
Certification dramatically enhances your portfolio, qualifying you for roles such as:
Lead Principal Investigator – Oversee complex, multinational trials.
Chief Medical Officer – Guide clinical strategy and trial design.
Academic Principal Investigator – Shape clinical research in academic institutions.
Certified PIs find opportunities in renowned CROs, hospitals, and academic settings, with ** top salaries of $279k+ annually** in senior positions.
Learn More About These Roles.
-
The CCRPS certification has been adopted by leading PI groups and major research organizations, further underscoring its importance. With certification, PIs gain access to roles such as:
Lead Principal Investigator – Direct multinational clinical trials with precision and authority.
Chief Medical Officer – Strategize and oversee clinical research operations.
Oncology, Neurology, or Cardiology Specialist PI– Excel in diverse therapeutic areas supported by niche expertise.
Certified PIs are highly valued for their ability to achieve efficiency, compliance, and superior trial outcomes, making them a trusted choice for sponsors globally. Salaries can exceed $279k in senior roles, while the certification provides the backing needed to lead prestigious trials.
Explore High-Demand Roles.
-
Set yourself apart in the competitive field of clinical research with the most recognized Principal Investigator certification. By completing the APIPC, you demonstrate a commitment to achieving excellence and mitigating risks for trial sponsors. Backed by on-demand videos, live webinars, and job placement services, along with flexible payment plans and a 14-day money-back guarantee, this program offers everything you need to succeed.
Enroll Today and join the growing network of certified PIs shaping the future of clinical research! -
Graduates obtained roles including: Principal Investigator in Clinical Research, Principal Research Investigator, Senior Principal Investigator, Clinical Trial Principal Investigator, Clinical Research Nurse Investigator, Oncology Principal Investigator, Radiation Therapy Principal Investigator, Academic Principal Investigator, Healthcare Settings Principal Investigator.
Advance your career with the Advanced Principal Investigator Physician Certification, designed for principal investigators and anyone seeking principal investigator training. Gain skills through 150 modules, covering advanced modules through pi training online as well as basics covering principal investigator definition and what does the principal investigator do.
Learn what’s the relationship of pi and co pi, explore principal investigator salary, and master pi research topics.
Principal Investigator Career Opportunities
This program prepares you for top positions such as principal research investigator, senior principal investigator, and radiation therapy principal investigator. Want to know what does the principal investigator do or explore principal investigator salarypotential? This certification sets you on a path to success.
Take the next step in your career with the Advanced Principal Investigator Physician Certification, and become a leader in clinical research. Enroll today.
Utilize Your Medical Knowledge with with Medical Monitor Certification
150 Advanced Modules I MD/MBBS with Prior Trials Exp I Trial Niches
"I was able to immediately apply what I learned.. to my job." - Rose Hyson
-
Medical Monitors are critical in clinical trials, ensuring patient safety, protocol compliance, and accurate data collection. They collaborate with sites, sponsors, and global trial teams to oversee trial integrity, regulatory adherence, and risk management. Certified Medical Monitors earn $49k–$110k+ annually, with top professionals earning more.
Learn More About Becoming a Medical Monitor -
Transition into a high-demand, nonclinical career with our 150-hour accredited program. Complete this training in just 2-4 weeks with self-paced learning. Select a pricing package tailored to your goals:
$499 – Course + Certification Exam.
$999 – Live Webinars + Job Support.
$1,499 – 1-1 Mentorship + Job Placement.
Explore Pricing and Register to start your career today.
-
Learn today’s most advanced methods, including:
Decentralized Trial Processes for global compliance.
Integration of patient engagement technologyand risk-based monitoring.
This training keeps you competitive in a rapidly evolving field.
Stay Ahead and Enroll Today
-
Master crucial leadership and technical skills, such as:
Building risk mitigation strategies.
Overseeing multi-site clinical trials with ease.
Managing regulatory submissions and data review.
Position yourself for high-paying roles, including Medical Director or Lead Medical Monitor.
Advance Your Career With Confidence
-
Explore rewarding career opportunities beyond clinical practice with our Medical Monitor Certification course. This program equips MDs with the skills and knowledge necessary to excel in nonclinical roles, such as:
Medical Writing to craft compelling regulatory documents, clinical trial reports, and educational materials.
Regulatory Affairs to ensure compliance with global guidelines and play a crucial role in drug and device approval processes.
Clinical Research Management to oversee pivotal trials, ensuring patient safety and data integrity.
Our course provides detailed training in areas like protocol development, risk management, and safety monitoring, ensuring you're prepared for transition into these high-demand roles. Take the next step in your career by building expertise tailored for growth in nonclinical settings.
-
Graduates obtained roles including: Clinical Research Medical Monitor, Medical Monitor, Principal Medical Monitor, Lead Medical Monitor, Clinical Trial Medical Monitor, Medical Oversight Director, Associate Medical Monitor, Senior Medical Monitor, Clinical Study Physician, Medical Advisor for Clinical Research, Clinical Research Physician, Medical Safety Monitor, Medical Director of Clinical Research,Drug Safety Medical Monitor, and more.
The Medical Monitor Certification is a specialized program designed to advance careers in clinical trial monitoring certification, medical monitor certification, and medication monitoring program.
Complete 250 on-demand modules in 2-4 weeks, earning 17.5 AMA- and ACCRE-accredited CME credits. Designed for professionals exploring nonclinical MD jobs, nonclinical physician jobs, nonclinical nursing jobs, and nonclinical jobs for physicians.
Priced from $499, with options for live sessions or mentorship at $999 and $1,499. Payment plans start at $33/month. Graduates secure roles like Clinical Research Physician, Medical Safety Monitor, and Senior Medical Monitor, earning $49k-$110k annually.
Clinical Research Organizations
Clinical Trials Staffing
CCRPS offers CROs a cost-effective staffing solution, saving $10,000–$50,000 monthly by eliminating recruiter fees and ensuring 5-day hiring turnaround. With a network of 25,888 pre-certified CRAs, CRCs, and regulatory specialists, CCRPS delivers job-ready candidates and provides free externship placement and long-term candidate development to enhance efficiency and clinical excellence.
Clinical Research Staff Training
CCRPS offers accredited clinical research training, ensuring compliance with FDA CFR 21, ICH GCP, and other regulations. Organizations can customize courses with SOP/MOP integration, track progress, and provide instant certifications. Discounts are available for groups over 10 participants, making it a cost-effective training solution for all staff levels.
Internship Partners
CCRPS delivers industry-recognized clinical research certifications accredited by ACCRE, AMA, ANCC, and aligned with ICH GCP standards. With 8+ years of success, CCRPS has trained professionals from over 1,200 organizations and 308 universities, placing graduates in top CROs. Organizations benefit from certified talent, streamlined recruitment, and externship programs to build a skilled workforce.
CCRPS Clinical Research Courses
1. ICH GCP Certification
Gain expertise in Good Clinical Practice, ensuring compliance with global research standards and actually understand how to apply
Who It’s For: All clinical professionals and researchers. Take every 2 years (free lifetime access).
Duration: 1 Week.
Career Outcomes: Clinical Research Assistant, Coordinator, Associate, or Regulatory Advisor.
2. Clinical Research Assistant Certification
Kickstart your career by mastering the fundamentals of clinical trials at an expert level.
Who It’s For: Entry-level professionals with an HS diploma or GED.
Duration: 3 Weeks.
Career Outcomes: Research Assistant or Clinical Trial Assistant, with salaries up to $70,000.
3. Clinical Research Coordinator (CRC) Certification
Learn expert-level skills to effectively run and coordinate clinical trials with full coverage of trial niches and more.
Who It’s For: Healthcare professionals or graduates with 2-year science degrees.
Duration: 4 Weeks.
Career Outcomes: Clinical Research Coordinator, earning $59,000–$80,000.
4. Clinical Research Associate (CRA) Certification
Advance into clinical research leadership roles with comprehensive monitoring training.
Who It’s For: Bachelor’s degree holders or current CRCs.
Duration: 4 Weeks
Career Outcomes: Roles like Clinical Research Associate or Trial Manager, earning up to $103,000+ annually.
5. Pharmacovigilance Certification
Master drug safety, adverse event reporting, and regulatory standards.
Who It’s For: Researchers and PharmD graduates.
Duration: 4 Weeks.
Career Outcomes: Pharmacovigilance Scientist or Drug Safety Manager, earning up to $120,000.
6. Clinical Project Manager Certification
Step into leadership with skills in project management and team coordination.
Who It’s For: Experienced trial professionals or project managers.
Duration: 4 Weeks.
Career Outcomes: Clinical Project Manager, earning $80,000–$160,000+.
7. Medical Monitor Certification
Take on advanced clinical trial safety roles and non-clinical MD opportunities.
Who It’s For: Physicians exploring non-clinical pathways.
Duration: 4 Weeks.
Career Outcomes: Medical Monitor or Clinical Research Physician, earning up to $110,000.
8. Principal Investigator (PI) Certification
Lead groundbreaking research studies as a Principal Investigator covering over 30 trial niches.
Who It’s For: MDs, PhDs, or active physicians.
Duration: 2-4 Weeks.
Career Outcomes: Roles like Principal Investigator or Senior Investigator.