Benefits of eConsent in Clinical Trials: A Modern Consent Solution
What is eConsent in Clinical Trials?
eConsent is the digital version of the traditional informed consent in a clinical trial. It replaces tedious paper based processes with digital consent forms and participants can read, understand and sign the documents electronically using a secure eConsent platform. This way, the process is simplified, the participant’s comprehension is improved and the clinical consent tracking is precise and accurate.
Clinical research is evolving and eConsent software for clinical trials is now the preferred way of consent management. Regulatory bodies like the FDA and EMA are also more and more in favor of digital consent solutions for clinical trials, meeting the ethical standards, improving the accessibility and compliance.
Check our blog to learn about reasons to adopt eConsent.
Why is eConsent Important for Clinical Trials in 2025?
With the development of decentralized and digital clinical trials, an eConsent platform for clinical trials has not become a nice thing to have—it has become a necessity to make sure all parties involved are protected. Modern eConsent solutions for clinical trials offer several advantages:
✔️ Improved patient accessibility – Thus eConsent clinical trial forms can be retrieved by participants from any location which makes it easier for them to participate in the study remotely.
✔️ Regulatory compliance – Using eConsent software clinical trials audit trails simplify compliance with GCP and ICH guidelines.
✔️ Enhanced transparency – Digital consent forms ensure participants fully understand study details.
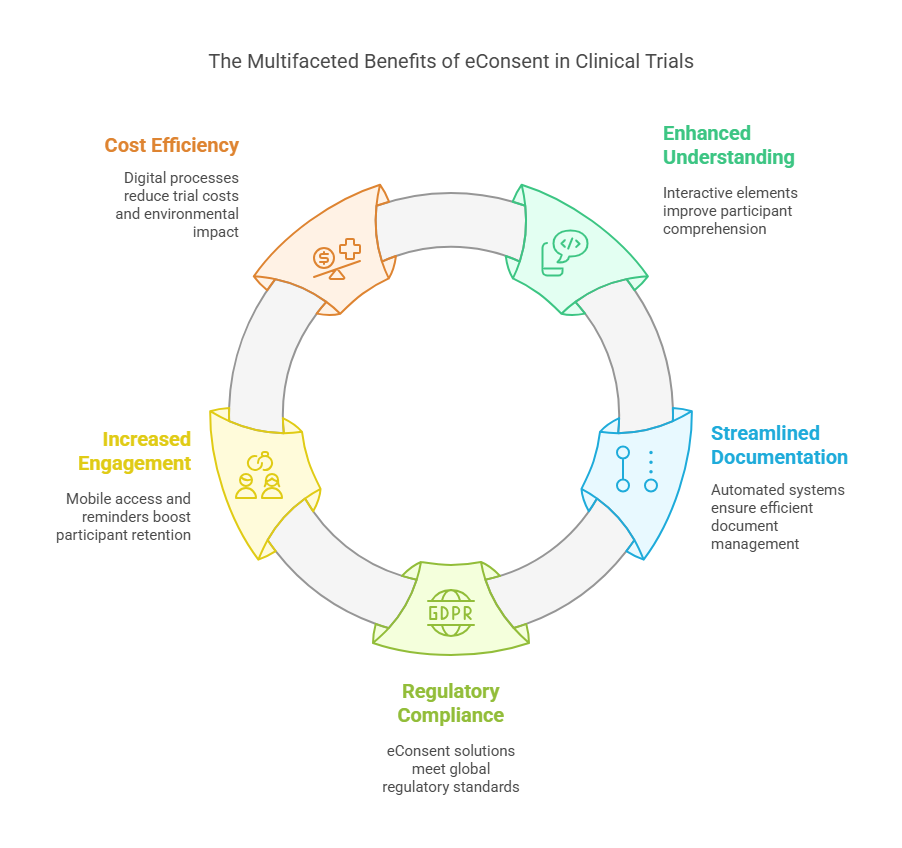
Key Benefits of eConsent in Clinical Trials
1. Enhanced Participant Understanding
A major challenge in clinical research is participant comprehension of study needs. Such as, eConsent in clinical trials introduces interactive elements, such as:
📌 Videos and animations – Help explain complex concepts visually.
📌 Multilingual support – Breaks language barriers in international trials.
📌 Real-time Q&A features – Participants can ask questions instantly.
According to a 2025 industry report, eConsent in clinical trials increase understanding by up to 40% thus decreasing the chances of misunderstanding and participant ambiguity.
2. Streamlined Documentation & Clinical Consent Tracking
Clinical research is inefficient with respect to managing paper documents and is error prone. Thus, with the use of eConsent software clinical trials, documentation is automated, secured and easily retrievable.
🔹 Cloud-based storage – Eliminates the risk of misplaced forms.
🔹 Real-time updates – Researchers track participant signatures instantly.
🔹 Automated compliance – Reduces regulatory risks.
Through the use of eConsent software in clinical trials, participants can be enrolled faster and trial documentation remains accurate and up to date for audits.
3. Regulatory Compliance & Ethical Standards
Regulatory bodies worldwide are pushing for more transparency in clinical trials. eConsent solutions provide:
✅ Regulatory adherence – FDA and EMA-compliant eConsent software for clinical trials.
✅ Audit-ready records – Digital timestamps track every step.
✅ Improved participant rights protection – Clearer disclosures reduce ethical concerns.
It is expected that by 2025 more than 75% of the global clinical trials will adopt eConsent digital trials which are consistent with the increasing importance of compliant eConsent solutions for clinical trials.
4. Increased Participant Engagement & Retention
High dropout rates have always been a challenge in clinical trials. eConsent for clinical trials helps retain participants through:
💡 Mobile-friendly access – Participants can sign from any device.
💡 Automated reminders – Ensures timely consent renewals.
💡 Transparent study details – Reduces uncertainty and confusion.
A recent study found that eConsent clinical trials achieve 30% higher retention rates than paper-based processes.
5. Cost-Efficient & Environmentally Friendly
Transitioning to eConsent platforms significantly reduces costs associated with:
📉 Paper and printing expenses
📉 Storage and manual tracking efforts
📉 Delays caused by misplaced or incomplete consent forms
Additionally, eConsent solutions for clinical trials contribute to sustainability efforts by minimizing paper waste.
How to Implement eConsent in Clinical Trials?
If you're considering eConsent for clinical trial implementation, follow these key steps:
Step 1: Choose a Secure eConsent Platform for Clinical Trials
Select an eConsent software clinical trials solution that meets:
✔️ FDA, EMA, and HIPAA compliance
✔️ User-friendly participant interface
✔️ Cloud security and encryption
Step 2: Train Clinical Teams
Educate your researchers on how to use the eConsent platform effectively. Ensure they understand consent tracking, compliance reporting, and participant interaction.
Step 3: Conduct a Pilot Study
Before full-scale deployment, test the clinical trial eConsent process with a small group of participants. Address any technical challenges or usability concerns.
Step 4: Provide Support & Accessibility Options
Offer training materials, FAQs, and 24/7 assistance to ensure participants understand the eConsent digital trials process.
The Future of Clinical Trial eConsent
With the rapid growth of digital healthcare, eConsent for clinical trials is set to become the gold standard for informed consent. The integration of AI-driven insights, blockchain for enhanced security, and seamless EHR (Electronic Health Records) integration will define eConsent solutions in the coming years.
By adopting a digital consent solution for clinical trials, research organizations like CCRPS can improve efficiency, compliance, and participant engagement—ultimately leading to faster and more reliable study outcomes.
Reference Links:
Johns Hopkins Medicine - Informed Consent in Clinical Trials
National Institutes of Health (NIH) - Ethical Considerations in eConsent
U.S. Food and Drug Administration (FDA) - eConsent Guidance for Industry
European Medicines Agency (EMA) - Electronic Systems in Clinical Trials
World Health Organization (WHO) - Guidelines on Informed Consent
Explore Courses for Clinical Research Career
Courses Available: