Top 5 Reasons to Adopt eConsent for Your Next Clinical Study
Clinical research has gradually become more sophisticated over the years and the application of technological advancements in this field has been helpful in improving the efficiency of processes as well as the participation of participants. One such development is the eConsent, a digital solution that is changing the way informed consent is obtained in clinical research. As the pharmaceutical industry shifts to digitalization, the adoption of eConsent is gradually becoming inevitable.
This blog will highlight the five best reasons you should consider using eConsent in your next clinical study. In recent years, clinical research has grown in complexity and the application of technology has greatly improved the conduct of studies and the experience of participants. Such a development is eConsent, a digital solution that is changing the way informed consent is obtained in clinical research. However, as the pharmaceutical industry shifts to digitalization, eConsent adoption is gradually becoming essential.
What is eConsent?
It is important to understand what eConsent includes before getting into the benefits. Electronic informed consent (eConsent) is the process of obtaining informed consent from clinical trial participants using digital platforms such as tablets, smartphones, or computers. Many times, the current process involving the use of paper based informed consent has been described as being cumbersome and time consuming with the possibility of long documents that may tend to overwhelm the participants. eConsent is a more efficient and user friendly, and an engaging way of explaining the risks and benefits of the study, and the procedures to follow.
Whereas, eConsent is far better than its paper-based counterpart in the sense that it uses various multimedia features, such as videos, animations, and audio, to help the participants fully understand what is being studied in the research they are about to participate in. Moreover, some eConsent platforms are equipped with real-time question-and-answer features, which means that participants can clear their doubts on the spot. Therefore, eConsent is not only more effective, but also more participant-friendly.
1. Enhances Participant Understanding and Retention
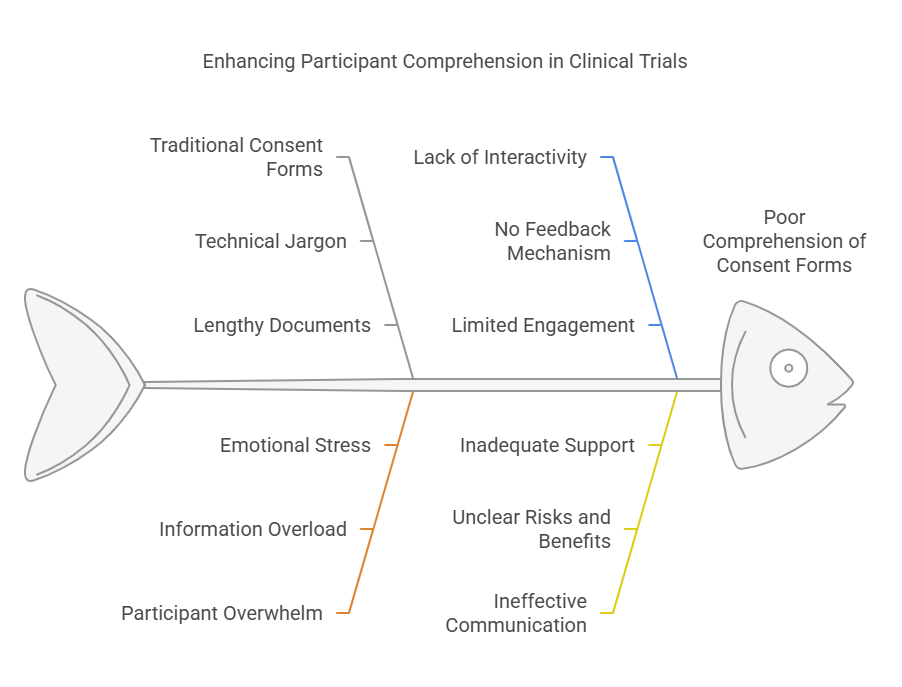
A major challenge in clinical trials is securing that participants fully comprehend the purpose, risks and benefits of the study before agreeing to enrol. Participants are often overwhelmed or confused by traditional, long, and technical jargon filled paper based consent forms. As a result, this often results in poor comprehension, which may limit a participant's capacity to make informed decisions.
The challenge of understanding consent is addressed by eConsent using various multimedia features including videos, infographics and voiceovers. Using such tools, it is easier to understand the medical information required that participants would be able to retain it better. Also, the interface has some interactive elements that enable the participants to interact with the material, to ask questions and to go back to certain parts if they need clarification. This results in a more informed and less apprehensive decision-making process.
Research has revealed that eConsent is better understood by participants than paper based forms. Such improvement in the participant’s comprehension is not only good for the participant but also the clinical trial sponsors since it minimizes the likelihood of protocol deviations owing to participant’s ambiguity on the expectations of the study.
2. Streamlines the Consent Process
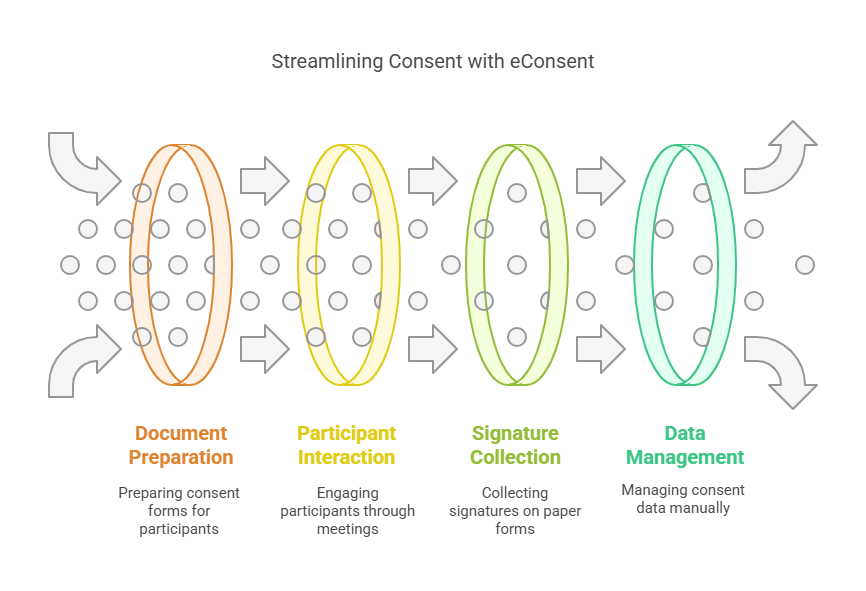
The conventional consent process can be lengthy, involving several steps like the printing of documents, one-on-one meetings and the usual signing of documents. This however becomes even more complicated in global studies where the participants are likely to be based in different regions of the world. Furthermore, paper consent forms have to be signed and kept which is time consuming and creates a lot of paperwork as well as the possibility of making mistakes.
Thus, eConsent eliminates most of the above mentioned inefficiencies. It is an electronic version of the documents that participants can read and sign the forms easily from the convenience of their homes, without the need to meet in person. This also speeds up the enrollment process but also improves the participant’s experience through the flexibility of the model. Moreover, it is able to work in integration with other clinical trial systems, and thus, extract and manage data effectively.
Thus, using eConsent during the research process, investigators can easily collect, retrieve, and analyze the signed consent forms of the participants, track their progress and ensure that the whole process is in compliance with the required regulations. It also reduces the chances of mistakes like missing signatures or using the wrong form which may slow down the study time line.
3. Improves Regulatory Compliance
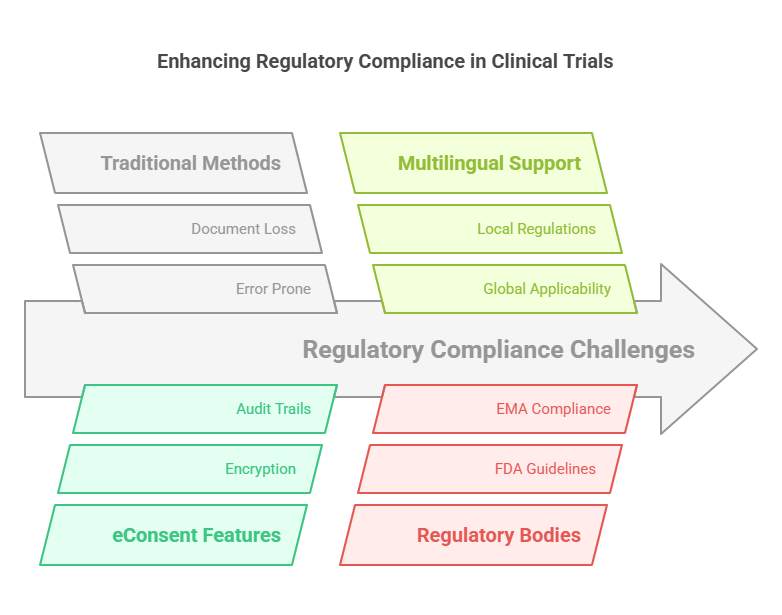
Clinical trials: Compliance with regulatory requirements is essential, particularly with respect to informed consent. The failure to comply with the guidelines put in place by regulatory bodies like the Food and Drug Administration (FDA) in the United States or the European Medicances Agency (EMA) can attract penalties, which may include fines or even the ending of a clinical trial. Traditional paper-based consent forms are also associated with compliance problems due to the possibilities of error, lost documents, or missing information.
Regulatory approval is built into eConsent platforms. Some features of many platforms are to make sure that all necessary information is provided and that participants can’t sign incomplete forms. Digital signatures are also securely encrypted and timestamped to validate authenticity and traceability. Additionally, eConsent systems can create audit trails yourself and do this automatically, which is a big help with inspections from regulatory authorities, for something that needs to be traceable.
Furthermore, eConsent platforms are able to provide multilingual options to help with the acquisition of informed consent from participants in different countries while the content of the eConsent remains compliant with the local regulations. This global applicability makes eConsent a valuable asset for multinational clinical trials.
4. Enhances Data Security and Privacy
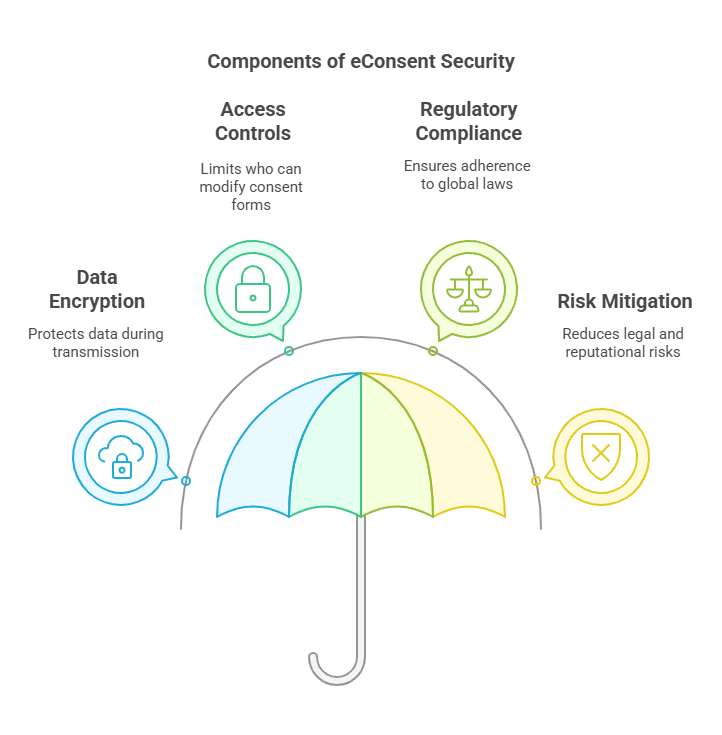
This is particularly important in clinical trials where participant information is sensitive in terms of data privacy and security. Paper based consent forms are easy to lose, steal or damage, thus putting the participant’s privacy at risk as well as the researchers’ legal liability.
On the other hand, eConsent platforms are security features that protect participant data. Consents forms and participant information are securely transmitted and stored using encryption protocols by these platforms. Access controls can also be used to limit who can access or change the consent forms, which provides another level of safety.
Moreover, eConsent systems are designed to comply with global data protection regulations such as the General Data Protection Regulation (GDPR) in the European Union and the Health Insurance Portability and Accountability Act (HIPAA) in the United States. This ensures that participant data is handled responsibly and ethically, reducing the risk of data breaches.
For clinical trial sponsors, the enhanced security features offered by eConsent platforms help mitigate the risk of non-compliance with data protection laws, which can result in hefty fines and damage to the organization’s reputation.
5. Boosts Participant Engagement and Retention
Participant recruitment and retention are two of the biggest challenges in clinical trials. Studies often experience high dropout rates, which can jeopardize the study's success. One of the key reasons participants drop out is due to a lack of understanding of the study’s procedures or feeling disconnected from the research process.
By adopting eConsent, researchers can improve participant engagement from the very beginning of the trial. The interactive nature of eConsent ensures that participants fully understand their role in the study and what is expected of them. This clarity helps build trust between the participant and the research team, leading to higher retention rates.
Additionally, eConsent platforms can be integrated with other digital tools, such as patient portals or mobile apps, that allow participants to stay informed throughout the study. Participants can receive real-time updates, reminders about appointments, and notifications about important study milestones. This ongoing communication helps participants feel more involved and reduces the likelihood of dropout.
Engaged participants are more likely to complete the study, providing researchers with more reliable data and reducing the need for costly recruitment efforts to replace dropouts.
Conclusion
The adoption of eConsent in clinical research is not just a trend—it’s a necessity in today’s digital landscape. From enhancing participant understanding to improving regulatory compliance, eConsent offers significant advantages that can boost the efficiency, security, and overall success of your clinical study. As the industry continues to shift toward digital transformation, integrating eConsent into your workflow will streamline the consent process and create a better experience for participants.
At CCRPS, we recognize the importance of modernizing clinical research practices. If you’re considering adopting eConsent for your next study, it’s crucial to choose a platform that aligns with your protocol requirements and regulatory standards. The benefits of switching to eConsent far outweigh the limitations of traditional paper-based methods—and early adoption can give your research the competitive edge it needs.
Reference Links:
U.S. Food & Drug Administration (FDA) - Informed Consent for Clinical Trials
National Institutes of Health (NIH) - eConsent and its Implementation in Clinical Trials
Clinical Trials Transformation Initiative (CTTI) - Best Practices for Digital Consent
World Health Organization (WHO) - Ethical Considerations in Clinical Trials
Explore Courses for Clinical Research Career
Courses Available: