Best Practices for Applying to Clinical Trials Vacancies in 2025
The clinical research industry continues to expand, offering numerous opportunities for professionals looking to advance their careers. The demand for skilled professionals in clinical trials is rising due to the increasing need for new therapies, vaccines, and medical technologies. However, with growing competition, it’s essential to know the best strategies to stand out when applying for these roles.
In this blog, we’ll explore key strategies for successfully applying to clinical trials vacancies in 2025. From crafting an effective resume to leveraging professional networks, these best practices will help you navigate the job application process and secure a rewarding role in clinical research.
Why Clinical Trials Jobs Are in High Demand?
Clinical trials play a crucial role in the development of new treatments and medical devices. These trials provide essential data on the effectiveness and safety of interventions, and as research becomes more complex, the need for qualified professionals has surged. The global clinical trials market is projected to exceed USD 70 billion by 2029, further increasing demand for skilled personnel.
As the industry grows, clinical trials vacancies are becoming more abundant. Positions range from clinical research associates (CRAs) and clinical research coordinators (CRCs) to data managers and principal investigators. Employers seek candidates who can ensure the smooth execution of trials while maintaining compliance with regulatory standards. However, securing a job in this field requires a strategic approach.
1. Understand the Types of Clinical Trials Vacancies Available
Before applying, it's important to familiarize yourself with the different clinical trials roles and their requirements. Each position demands unique skills and qualifications, so aligning your experience with the right job is crucial.
Common Clinical Trials Roles:
Clinical Research Coordinator (CRC) – Manages day-to-day trial operations, interacts with patients, and ensures studies follow protocols.
Clinical Research Associate (CRA) – Conducts site visits, monitors compliance with study protocols, and ensures accurate data collection.
Data Manager – Oversees the collection, storage, and analysis of clinical trial data, requiring a high level of accuracy.
Principal Investigator (PI) – Leads the clinical trial, ensuring adherence to regulatory and ethical guidelines while overseeing the research team.
Regulatory Affairs Specialist – Ensures clinical trials comply with government regulations and industry standards.
Understanding these roles will help you tailor your application to highlight the most relevant skills and experiences.
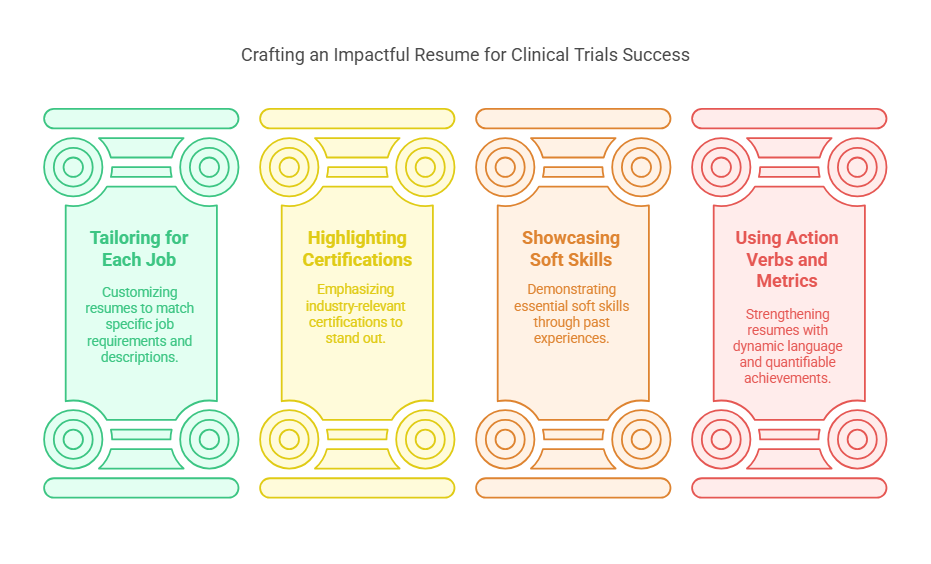
2. Crafting a Winning Resume for Clinical Trials Vacancies
Your resume is your first opportunity to make a strong impression on hiring managers. To increase your chances of landing an interview, focus on these key elements:
Tips for a Standout Resume:
Tailor Your Resume for Each Job – Customize your resume for each role by emphasizing relevant experience and skills that match the job description.
Highlight Industry Certifications – Certifications such as Good Clinical Practice (GCP), Clinical Research Associate (CRA), or Clinical Research Coordinator (CRC) certifications can set you apart from other applicants.
Showcase Soft Skills – Clinical trials roles require strong communication, problem-solving, and organizational skills. Demonstrate how you’ve applied these abilities in past roles.
Use Action Verbs and Metrics – Strengthen your resume with action verbs like “coordinated,” “managed,” or “analyzed.” Include metrics when possible, such as “Managed a trial involving 200+ participants across three locations.”
By presenting a clear and well-structured resume, you increase your chances of capturing an employer’s attention.
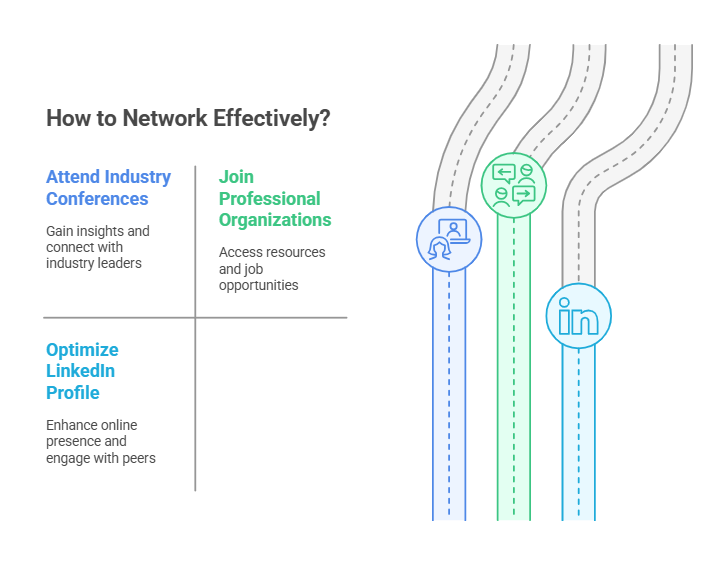
3. Leverage Networking Opportunities in the Clinical Trials Industry
Many clinical trials jobs are filled through professional connections. Building and maintaining a strong network can open doors to job opportunities that may not be publicly advertised.
How to Network Effectively:
Attend Industry Conferences – Events like the SOCRA Annual Conference and ACRP Global Conference provide networking opportunities and insights into industry trends.
Join Professional Organizations – Membership in organizations such as the Association of Clinical Research Professionals (ACRP) or Society of Clinical Research Associates (SOCRA) gives access to job boards, webinars, and training resources.
Optimize Your LinkedIn Profile – Keep your profile updated with relevant experience, certifications, and industry keywords. Engage with industry professionals and follow clinical research organizations.
Building a strong professional network can give you a competitive edge in the clinical trials job market.
4. Stay Updated on Industry Trends and Requirements
The clinical trials landscape is continuously evolving. Staying informed about the latest industry trends can enhance your employability and demonstrate your commitment to the field.
Key Trends to Watch in 2025:
Decentralized Clinical Trials (DCTs) – The rise of virtual and hybrid trial models requires professionals with experience in remote monitoring and digital health technologies.
eConsent and Digital Tools – Familiarity with electronic consent processes and digital data collection methods is becoming increasingly valuable.
Artificial Intelligence (AI) in Research – AI is playing a growing role in trial design, patient recruitment, and data analysis, making technical proficiency an asset.
Staying up-to-date through industry publications, online courses, and webinars will position you as a strong candidate for emerging roles.
5. Prepare for the Interview Process
Once your application stands out, the next step is preparing for interviews. Employers look for candidates who not only have the technical knowledge but also demonstrate problem-solving abilities and a commitment to compliance and ethics.
Interview Preparation Tips:
Review Key Regulations – Be familiar with ICH-GCP guidelines, FDA regulations, and other relevant compliance requirements.
Practice Common Questions – Prepare for questions about your clinical research experience, problem-solving abilities, and how you handle challenges in trial management.
Showcase Your Achievements – Use the STAR method (Situation, Task, Action, Result) to highlight specific examples of your contributions in past roles.
Confidence and preparation will help you make a lasting impression during the interview process.
Reference Links:
National Institutes of Health (NIH) - Clinical Trials and You
World Health Organization (WHO) - Clinical Trials
CenterWatch - Clinical Research Industry News
Conclusion
The demand for clinical trials professionals is set to rise in 2025, creating exciting career opportunities. By understanding the available roles, optimizing your resume, leveraging networking opportunities, staying informed about industry trends, and preparing for interviews, you can position yourself for success.
For those looking to enhance their qualifications, organizations like CCRPS (Certified Clinical Research Professionals Society) offer valuable certifications and training programs that can strengthen your expertise and improve your employability in the clinical research field.
Explore Courses for Clinical Research Career
Courses Available: