Clinical Laboratory Practice Certification
Getting Good Clinical Laboratory Practice (GCLP) certification is an important part of the clinical research and laboratory management. It guarantees that laboratories are run in a regulated manner to ensure data accuracy and reliability. Not only does this certification help to build the reputation of a laboratory but it also has a significant role in protecting the public health by helping to guarantee the accuracy of clinical trials and research.
What is GCLP?
A combination of Good Laboratory Practice (GLP) and Good Clinical Practice (GCP) principles is captured by Good Clinical Laboratory Practice (GCLP), to guarantee that laboratories supporting clinical trials deliver accurate, consistent and of good quality data. GCLP guidelines extend to all aspects of laboratory activities, including sample collection, analysis, data documentation and reporting.
Importance of GCLP Certification
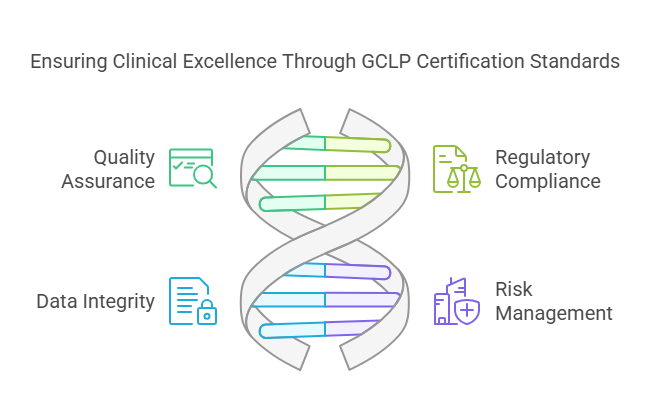
GCLP certification is vital for several reasons:
Quality Assurance: To ensure that laboratory processes and data are consistent and reproducible, this standard must be met.
Regulatory Compliance: Meets the regulatory requirements set by authorities like the FDA and EMA.
Data Integrity: Protects the accuracy and completeness of data used in clinical research.
Risk Management: Minimizes the chances of mistakes and violations that can result in expensive delays or failures in clinical trials.
Benefits of GCLP
There are following benefits of GCP:
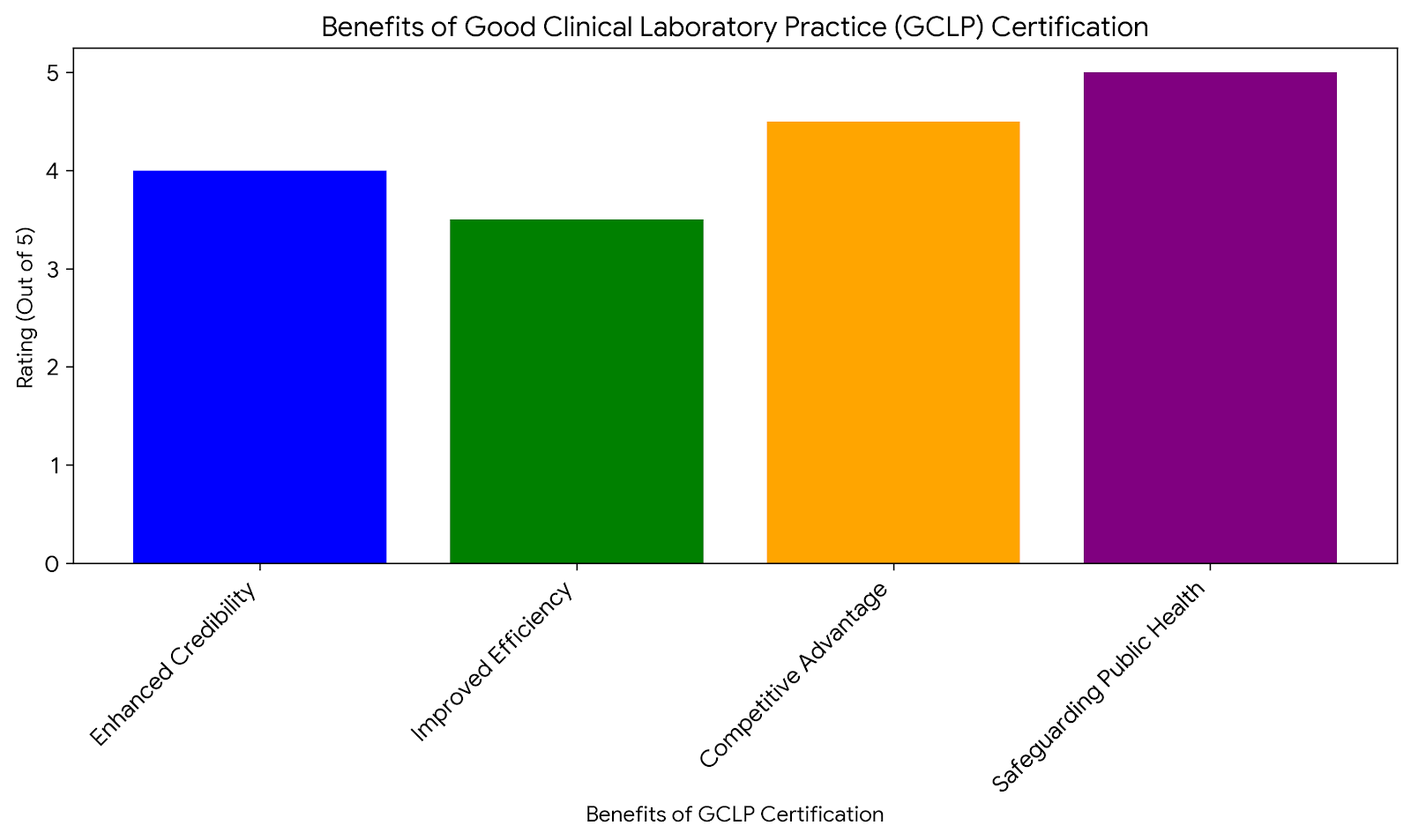
Enhanced Credibility and Trust
Having GCLP certification does enhance the credibility of a laboratory to some extent. It shows commitment to high standards and develops sponsor, regulatory, and public trust.
Improved Laboratory Efficiency
Implementing GCLP guidelines streamlines laboratory processes, leading to increased efficiency and productivity. Standardized procedures reduce the likelihood of errors, saving time and resources in the long run.
Competitive Advantage
GCLP-certified laboratories are often preferred by sponsors and clients in the pharmaceutical and biotechnology industries. This certification can provide a competitive edge, attracting more business opportunities.
Safeguarding Public Health
By ensuring the accuracy and reliability of clinical data, GCLP certification plays a crucial role in safeguarding public health. Reliable data is essential for developing effective treatments and therapies.
Key Components of GCLP Certification
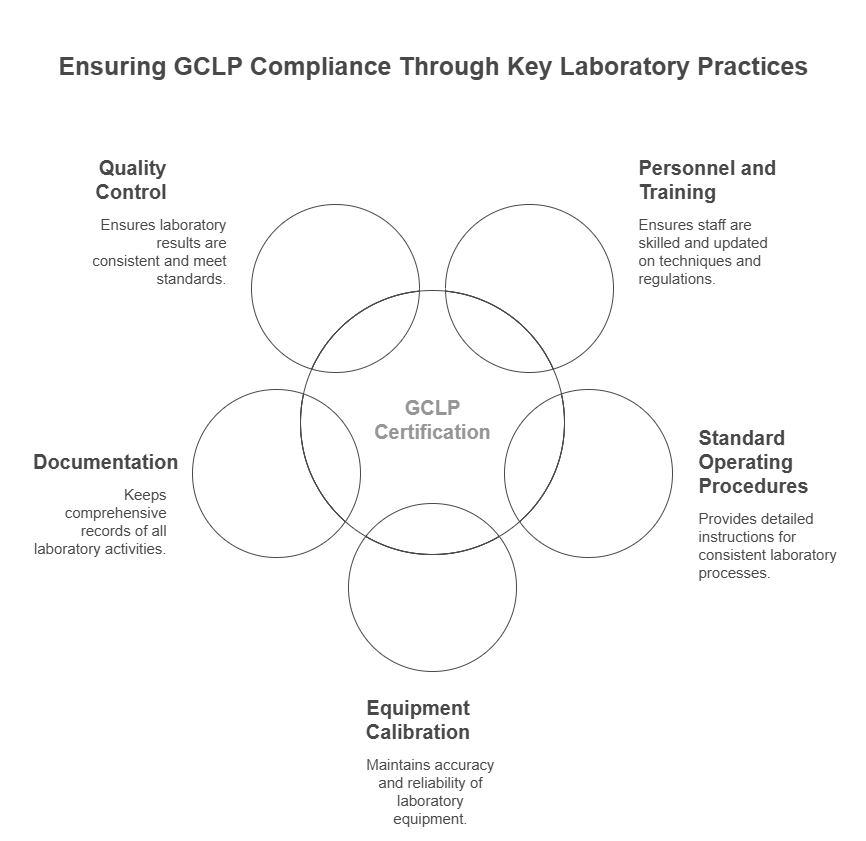
Personnel and Training
Ensuring that laboratory personnel are adequately trained is a cornerstone of GCLP certification. Continuous training programs keep staff updated on the latest techniques and regulatory requirements.
Standard Operating Procedures (SOPs)
SOPs are detailed, written instructions to achieve uniformity in the performance of a specific function. They are crucial for maintaining consistency and quality in laboratory processes.
Equipment Calibration and Maintenance
Regular calibration and maintenance of laboratory equipment are essential to ensure accurate and reliable results. GCLP guidelines require documented proof of equipment performance.
Documentation and Records
Accurate documentation is fundamental to GCLP compliance. This includes detailed records of all laboratory activities, from sample collection to data analysis and reporting.
Quality Control and Assurance
Implementing robust quality control measures ensures that laboratory results are consistent and reliable. Quality assurance processes verify that all aspects of laboratory operations meet the required standards.
Implementing GCLP in Your Laboratory
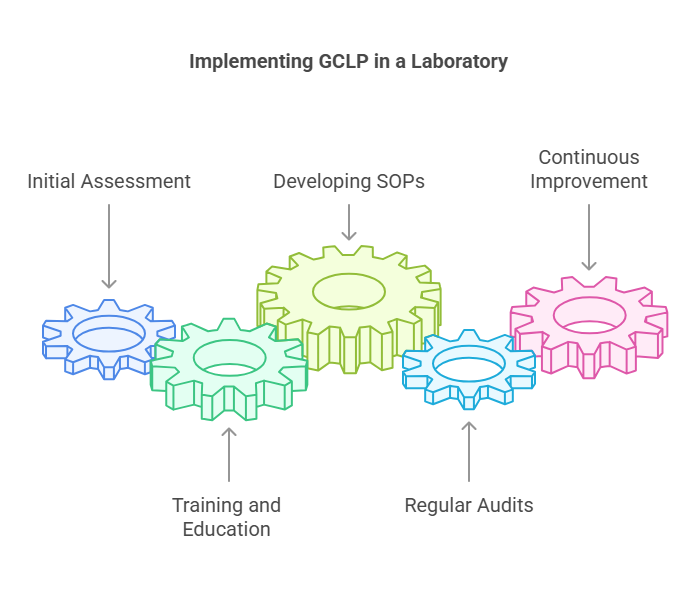
Initial Assessment
Conducting an initial assessment of current laboratory practices against GCLP standards is the first step towards implementation. This helps identify gaps and areas for improvement.
Training and Education
Investing in comprehensive training programs for laboratory staff is crucial. This ensures that everyone is aware of GCLP guidelines and understands their role in maintaining compliance.
Developing SOPs
Creating detailed SOPs for all laboratory processes is essential. These should be regularly reviewed and updated to reflect any changes in practices or regulations.
Regular Audits
Conducting regular internal and external audits helps ensure ongoing compliance with GCLP standards. Audits can identify potential issues before they become significant problems.
Continuous Improvement
GCLP implementation is an ongoing process. Continuous improvement initiatives, such as feedback loops and process reviews, help maintain high standards and adapt to new challenges.
The Impact of GCLP Certification on Clinical Research
Ensuring Data Integrity
One of the most significant impacts of GCLP certification is the assurance of data integrity. Reliable data is the foundation of clinical research, and GCLP ensures that data is accurate, consistent, and reproducible.
Enhancing Patient Safety
By maintaining high standards of laboratory practice, GCLP certification enhances patient safety. Accurate and reliable data is crucial for developing safe and effective treatments.
Facilitating Regulatory Approval
GCLP-certified laboratories are more likely to meet the stringent requirements of regulatory authorities. This can expedite the approval process for new treatments and therapies, bringing them to market faster.
Boosting Research Efficiency
Standardized procedures and robust quality control measures streamline laboratory operations, leading to increased efficiency and productivity in clinical research.
Laboratory Information Management Systems (LIMS)
Laboratory Information Management Systems (LIMS) play a vital role in achieving GCLP compliance. These systems automate data collection, management, and reporting, ensuring accuracy and traceability.
Automation and Robotics
The integration of automation and robotics in laboratory processes reduces the likelihood of human error and increases efficiency. Automated systems can handle repetitive tasks, freeing up staff for more complex activities.
Data Analytics and AI
Advanced data analytics and artificial intelligence (AI) tools can enhance data analysis and interpretation, leading to more accurate and insightful results. These technologies support GCLP compliance by ensuring data integrity and reliability.
Evolving Standards and Guidelines
As scientific research and technology continue to advance, GCLP standards and guidelines will evolve. Staying updated with these changes is essential for maintaining compliance and ensuring the highest quality of laboratory practice.
Global Harmonization
Efforts towards global harmonization of GCLP standards are underway. This will facilitate international collaboration and streamline the regulatory approval process for new treatments and therapies.
Emphasis on Data Security
With the increasing amount of data generated in clinical research, data security will become a more significant focus. GCLP guidelines will likely include more stringent requirements for protecting sensitive information.
Conclusion
Good Clinical Laboratory Practice (GCLP) certification is a cornerstone of quality and reliability in clinical research. By ensuring high standards of laboratory practice, GCLP certification enhances credibility, efficiency, and data integrity. Implementing GCLP guidelines is an ongoing process that requires continuous improvement and adaptation to new challenges. As technology and standards evolve, staying updated with GCLP requirements will be crucial for maintaining compliance and ensuring the highest quality of clinical research.
References
ICH GCP Guidelines - ICH Official Website
FDA Guidelines on GCLP - FDA Official Website
EMA GCP Compliance - EMA Official Website
WHO GCLP Guidelines - WHO Official Website
Laboratory Information Management Systems (LIMS) - LabWare
Quality Assurance in Clinical Laboratories - Journal of Clinical Laboratory Analysis
Explore Courses for Clinical Research Career
Courses Available: