How Clinical Trials Work: A Beginner’s Guide
Medical progress depends on clinical trials to validate the safety and effectiveness of new treatments before their public availability. This detailed guide explains the clinical trial stages together with governing protocols and safety protocols and ethical aspects and it includes information about recent advancements up to 2025.
What Are Clinical Trials?
Clinical trials serve as research studies which evaluate medical and behavioral interventions through human participant involvement. Researchers depend on clinical trials as their main approach to establish whether new treatments including drugs and medical devices are safe and effective for human use.
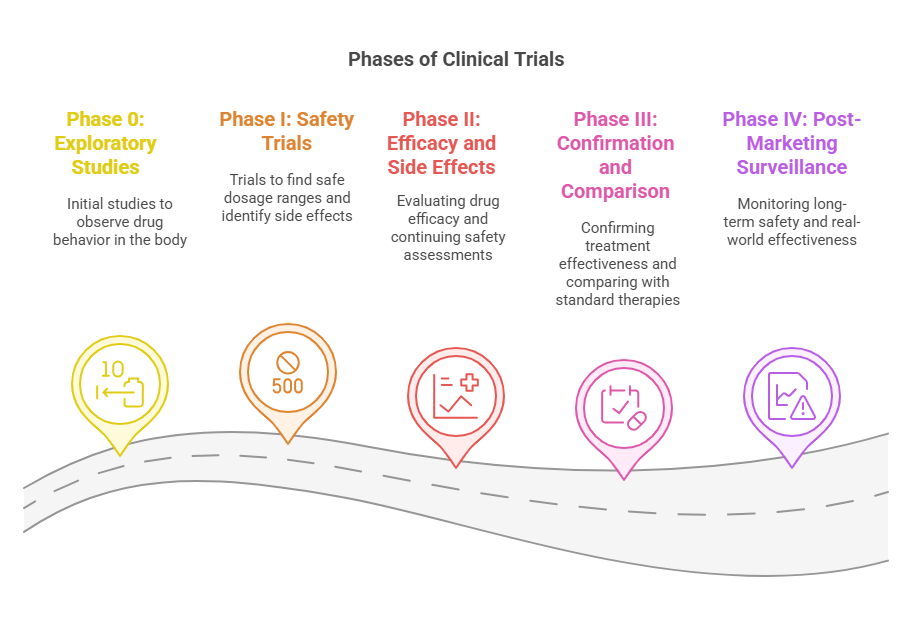
The Phases of Clinical Trials:
Medical interventions undergo clinical testing through sequential phases which evaluate their safety and both immediate and extended effects. The phases of clinical trials serve unique objectives which expand upon previous study findings. The phases operate under strict regulatory guidelines that maintain both scientific excellence and ethical compliance.
🔬 Phase 0: Exploratory Studies (First-in-Human Trials)
The main goal is to see how the new drug behaves in the body — especially how it is absorbed, distributed, metabolized, and excreted. This is also known as studying the pharmacokinetics and pharmacodynamics.
👥 Participants:
10–15 healthy individuals or patients with the disease, depending on the therapy.
🔍 Focus Areas:
Pharmacokinetics (PK): How the body processes the drug
Pharmacodynamics (PD): How the drug affects the body
Establishing early safety data before larger trials
🧪 Phase I: Safety Trials
The researchers will use this phase to establish a safe dosage range and monitor any side effects that may occur. This phase also helps to understand how the drug behaves inside the human body in real time.
👥 Participants:
20–80 healthy volunteers or patients (in cancer trials, patients are often recruited due to the risks of placebo in terminal conditions).
🔍 Focus Areas:
Establishing maximum tolerated dose (MTD)
Identifying adverse reactions
Determining pharmacokinetics (absorption, metabolism, etc.)
Route of administration: oral, intravenous, etc.
✅ In 2025, Phase I trials increasingly involve AI-powered modeling to predict reactions before actual testing.
🧬 Phase II: Efficacy and Side Effects
To determine if the treatment is effective for a particular condition, and to monitor safety and adjust dosing.
👥 Participants:
100–300 individuals with the disease or condition being studied.
🔍 Focus Areas:
Efficacy: Does the drug do what it’s supposed to?
Side Effects: Are they mild, moderate, or severe?
Dosing Schedules: Once a day vs. twice, etc.
✅ Many drugs fail in Phase II. In 2025, adaptive designs allow for early adjustments to improve success rates.
🧪 Phase III: Confirmation and Comparison
To confirm the treatment’s effectiveness, compare it with standard therapies, and gather detailed information on risks and benefits.
👥 Participants:
1,000–3,000 patients from diverse geographic and demographic backgrounds.
🔍 Focus Areas:
Comparison with existing treatments or placebo
Monitoring for less common side effects
Building the final evidence needed for regulatory approval
Design:
Often randomized, double-blind, placebo-controlled to reduce bias.
Significance:
Most expensive and time-consuming phase
Data is used for submission to FDA, EMA, and other authorities for approval
✅ By 2025, Phase III trials are adopting wearable tech and ePROs (electronic patient-reported outcomes) to gather real-time data from participants remotely.
🧾 Phase IV: Post-Marketing Surveillance
To monitor long-term safety and real-world effectiveness after the drug is approved and available to the public.
👥 Participants:
Thousands to millions from the general patient population.
🔍 Focus Areas:
Detecting rare or long-term side effects
Observing outcomes in specific populations (elderly, pregnant women, etc.)
Ensuring continued effectiveness under real-world conditions
Regulatory Oversight:
FDA requires manufacturers to submit periodic safety updates
Health providers report adverse events via programs like MedWatch
✅ In 2025, real-world data (RWD) and real-world evidence (RWE) are essential for ongoing approvals and insurance reimbursements.
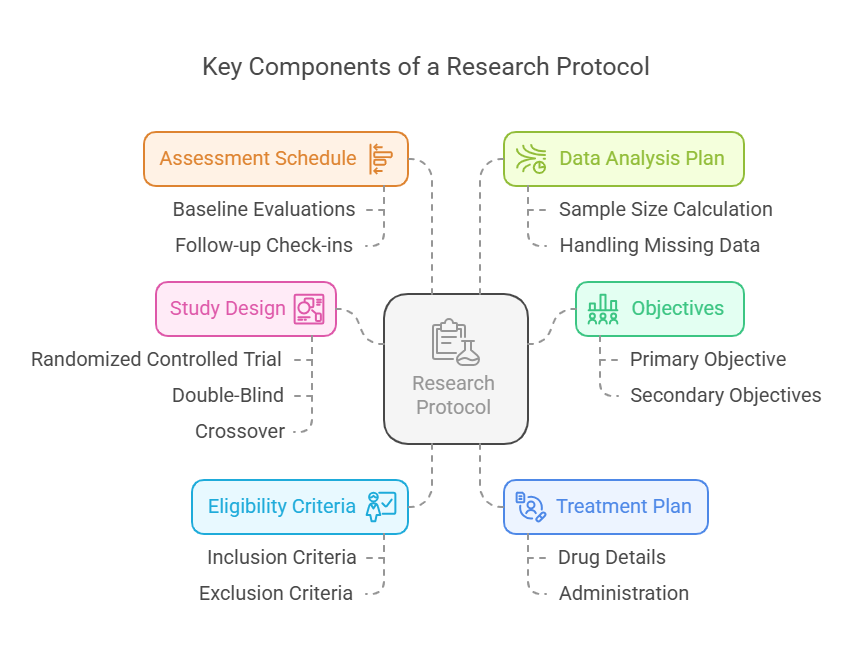
Understanding Research Protocols in Clinical Trials
A research protocol is the backbone of any clinical trial. It is a comprehensive document that outlines how a study will be conducted, ensuring that the research is scientifically sound, ethically compliant, and safely managed.
Just like a blueprint guides the construction of a building, a research protocol provides step-by-step instructions for researchers, investigators, and sponsors to follow. This guarantees that every participant, no matter where they are in the world, is treated consistently and fairly throughout the trial.
🧩 Key Components of a Research Protocol
Let’s break down the essential parts of a modern research protocol and why each one matters:
🎯 1. Objectives
Every trial must begin with clear primary and secondary objectives.
Primary Objective: The main question the study wants to answer.
Example: Does Drug A lower blood pressure more effectively than placebo?
Secondary Objectives: Additional questions that provide supportive or exploratory data.
Example: What is the effect of Drug A on cholesterol levels?
2025 Note:
More trials now use patient-centered objectives and include quality-of-life (QoL) outcomes as part of the research goals.
🧪 2. Study Design
Study design defines how participants will be grouped, treated, and evaluated. The design influences how reliable and unbiased the results will be.
Common types include:
Randomized Controlled Trial (RCT): Participants are randomly assigned to groups.
Double-Blind: Neither participants nor investigators know who gets the treatment or placebo.
Crossover: Participants receive multiple treatments in a specific order.
Why it matters:
A strong design minimizes bias and ensures that differences in outcomes are truly due to the intervention.
2025 Note:
Modern protocols often include adaptive designs, allowing changes mid-study based on interim data without compromising integrity.
✅ 3. Eligibility Criteria
This defines the inclusion and exclusion criteria, helping researchers recruit participants who are suitable for the study and reduce risks.
Inclusion Criteria:
Age, gender, disease stage, lab values, etc.
Helps define the target population for the drug.
Exclusion Criteria:
Factors that may interfere with the study or endanger the participant (e.g., pregnancy, allergies, other medical conditions).
Why it matters:
Ensures patient safety and that results are valid for the intended population.
💉 4. Treatment Plan
This section lays out the exact medical intervention participants will receive.
Includes:
Drug name and formulation
Dosage and administration route (oral, injection, etc.)
Frequency and duration of treatment
Any control or placebo protocols
Why it matters:
Consistency in treatment administration ensures data reliability and helps identify real drug effects.
📅 5. Assessment Schedule
This outlines what tests and evaluations will be done, and when.
Baseline evaluations (e.g., blood tests, imaging)
Follow-up check-ins
Adverse event monitoring
Final assessments
2025 Update:
With the rise of remote clinical trials and wearable tech, many assessments can now be done at home, improving convenience and compliance.
📊 6. Data Analysis Plan
Statistical methods are used to determine whether the results are significant and not due to chance.
Includes:
Sample size calculation (how many participants needed)
Handling of missing data
Primary endpoint analysis
Safety and subgroup analysis
Why it matters:
This ensures that the conclusions drawn are statistically valid and scientifically trustworthy.
Ethical Considerations in Clinical Trials
Ethics are paramount in clinical research to protect participants' rights and well-being. Key ethical principles include:
Informed Consent: Participants must be fully informed about the study and voluntarily agree to participate.
Risk-Benefit Assessment: Ensuring that the potential benefits justify any risks involved.
Confidentiality: Protecting personal information of participants.
Independent Review: Ethics committees or Institutional Review Boards (IRBs) review and approve study protocols.
Ethics committees play a crucial role in overseeing trials to ensure compliance with ethical standards and regulatory requirements.
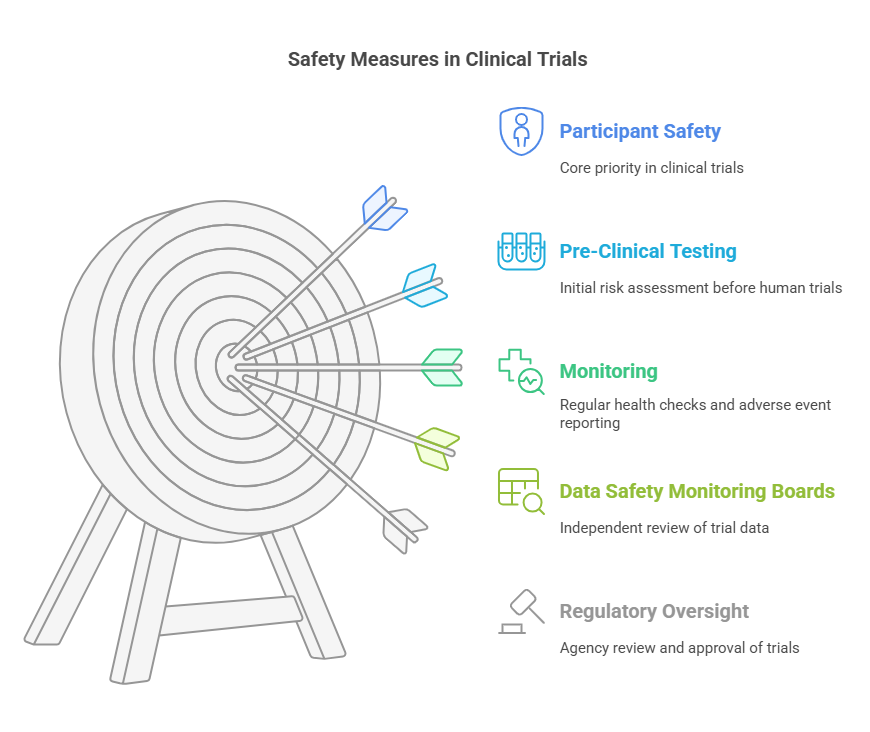
Ensuring Safety in Clinical Trials
Participant safety is a top priority throughout all phases of a clinical trial. Measures include:
Pre-Clinical Testing: Laboratory and animal studies before human trials.
Monitoring: Regular health assessments and reporting of adverse events.
Data Safety Monitoring Boards (DSMBs): Independent groups that review data and ensure ongoing safety.
Regulatory Oversight: Agencies like the FDA review trial data before approving new treatments.
These measures help identify risks early and protect participants from harm.
Lesser-Known Facts About Clinical Trials
Certain populations, including women and minorities, are significantly underrepresented in clinical trials. For example, women represent less than 40% of participants in heart disease and stroke research, despite accounting for a larger proportion of patients affected by these conditions. This lack of diversity impacts the generalizability of trial results and may lead to unforeseen side effects in underrepresented groups. (Source)
The journey from initial research to public availability of a new treatment can take 10–15 years. This timeline includes preclinical research, multiple trial phases, and regulatory approval, with clinical trials alone often spanning six to seven years. (Source)
Developing a new drug through clinical trials is an expensive endeavor, with costs averaging $1 billion. This figure includes expenses for failed projects and highlights the financial risk inherent in drug development. (Source)
Placebos are commonly used in clinical trials to compare the effectiveness of new treatments. Ethical guidelines ensure that participants are not denied necessary care, especially when effective therapies already exist. (Source)
Clinical trials are conducted worldwide, with countries like China increasing their share significantly in recent years. This global collaboration accelerates research but also highlights disparities in trial accessibility across regions. (Source)
Conclusion
Understanding how clinical trials work is essential not only for medical professionals and researchers but also for patients and caregivers considering participation. From early exploratory studies to large-scale post-marketing evaluations, each phase plays a critical role in ensuring that new treatments are safe and effective.
By following strict research protocols, prioritizing patient safety, and maintaining ethical standards, clinical trials help bring innovation to healthcare in a responsible way.
For trustworthy clinical trial education and certification, CCRPS (Certified Clinical Research Professional Society) offers comprehensive training programs designed for professionals entering or advancing in the field.
Frequesntly Asked Questions (FAQs)
-
Clinical trials test new drugs, treatments, medical devices, or diagnostic tools to assess their safety and effectiveness in humans before they are approved for general use.
-
Eligibility is determined by specific criteria set in the study protocol. These include age, medical condition, previous treatments, gender, and overall health status.
-
Clinical trials are designed to prioritize patient safety. They must be approved by ethical review boards and follow strict guidelines. While risks exist, participants are closely monitored throughout.
-
Informed consent is a process where participants are fully educated about the trial’s purpose, procedures, risks, and benefits before agreeing to take part. This ensures transparency and voluntary participation.
-
esults help determine whether a new treatment is safe and effective. Positive findings may lead to regulatory approval by authorities like the FDA, EMA, or other international agencies.