Who Should Consider Clinical Research Training?
Clinical research is a vital component of medical advancements, bridging the gap between laboratory discoveries and patient care. It encompasses the systematic investigation of medical interventions, treatments, and devices to ensure their safety and efficacy. As the healthcare landscape evolves, the demand for skilled professionals in clinical research continues to grow. This article explores who should consider clinical research training, highlighting target audiences such as pharmacists, doctors, nurses, and science graduates.
Understanding Clinical Research
Clinical research is the foundation of modern medicine. It refers to a branch of healthcare science that focuses on evaluating medical, surgical, and behavioral interventions using human participants. The aim is to gather high-quality, evidence-based data to answer specific health-related questions and improve patient care.
🔍 Types of Clinical Research:
Clinical research isn’t limited to drug testing. It includes various forms such as:
Treatment Research – Studies on new drugs, therapies, or combinations of treatments.
Prevention Research – Investigates ways to prevent illnesses or their recurrence.
Diagnostic Research – Develops better methods to diagnose conditions accurately.
Screening Research – Identifies ways to detect diseases at earlier stages.
Quality of Life Research – Focuses on improving comfort and life for those with chronic conditions.
Genetic Studies – Looks at how genes influence health and illness.
Epidemiological Studies – Explore patterns, causes, and control of disorders in groups.
🔄 Clinical Trials: A Vital Subset
Clinical trials are a specific form of clinical research involving human volunteers (participants) who follow a strict protocol. These are typically conducted in four phases:
Phase I: Small groups (20–100 people) receive the treatment to assess safety and dosage.
Phase II: Larger groups test the treatment’s effectiveness and monitor side effects.
Phase III: Hundreds or thousands of participants are enrolled to confirm effectiveness, monitor side effects, and compare it to standard treatments.
Phase IV: After approval, studies continue to collect information on long-term effects and real-world use.
👩🔬 Why Human Participation?
Because animals or simulations can’t fully replicate human biology, human-based trials are essential. They help identify how real people react to new treatments in real-world scenarios.
🧷 Ethics and Oversight
All clinical research involving humans must follow Good Clinical Practice (GCP) guidelines and be approved by ethics committees or Institutional Review Boards (IRBs). These safeguards ensure participant safety, privacy, and informed consent.
📊 Outcomes of Clinical Research:
New drugs or medical devices getting approved (e.g., mRNA vaccines).
Improved diagnostic tools (e.g., liquid biopsies).
Better understanding of rare diseases and conditions.
Development of public health policies (e.g., cancer screening guidelines).
Why Clinical Research Training is Essential?
In an era of rapidly evolving medicine and increasing regulatory scrutiny, clinical research training is no longer optional—it's essential for professionals aspiring to work in this domain.
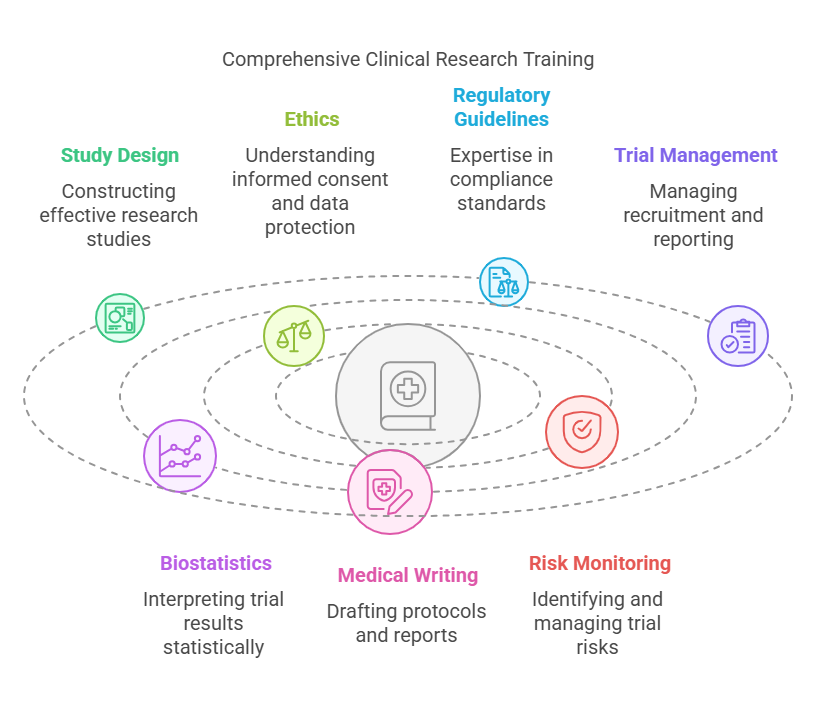
📘 What Does the Training Cover?
Study Design & Protocol Development
Learn how to construct effective, ethical, and scientifically valid research studies.Ethics in Research
Understand informed consent, participant rights, and data protection.Regulatory Guidelines
Gain expertise in ICH-GCP, FDA, EMA, and regional compliance standards.Clinical Trial Management
Study recruitment, monitoring, data collection, site coordination, and reporting.Biostatistics and Data Analysis
Interpret trial results using proper statistical methods.Medical Writing and Documentation
Learn to draft trial protocols, informed consent forms, clinical study reports, and regulatory submissions.Risk-Based Monitoring
Focuses on identifying and managing potential risks during trials to improve efficiency.
⚙️ Why Is This Training Crucial?
✅ Ensures Patient Safety: Trained professionals can identify adverse effects early and respond appropriately, reducing harm to participants.
✅ Meets Legal and Ethical Requirements: Global clinical research is highly regulated. Non-compliance can result in severe legal, financial, and reputational consequences.
✅ Boosts Research Integrity: Trained personnel are more likely to collect, analyze, and report data accurately—leading to trustworthy results.
✅ Opens Diverse Career Paths:
Training qualifies professionals for roles like:
Clinical Research Associate (CRA)
Clinical Data Manager
Regulatory Affairs Specialist
Trial Coordinator
Medical Writer
Clinical Operations Manager
✅ Speeds Up Medical Innovation: Well-managed trials get treatments to market faster, helping patients access life-saving drugs sooner.
Target Audiences for Clinical Research Training
1. Pharmacists
Pharmacists possess a deep understanding of drug composition, mechanisms, and interactions. This expertise positions them uniquely to contribute to clinical research, particularly in drug development and pharmacovigilance. By undergoing clinical research training, pharmacists can:
Participate in Clinical Trials: Engage in the design and execution of trials, ensuring appropriate medication management.
Ensure Regulatory Compliance: Navigate the complex landscape of drug approval processes and regulatory affairs.
Advocate for Patient Safety: Monitor adverse drug reactions and ensure ethical dispensing practices.
In Australia, for instance, pharmacists are now able to use the title "Doctor of Pharmacy" after completing additional study, reflecting the expanding scope of their roles in healthcare.
2. Doctors
Physicians are at the forefront of patient care, making their involvement in clinical research crucial. Training enables doctors to:
Lead Research Initiatives: Serve as principal investigators, overseeing study design and implementation.
Translate Research into Practice: Apply findings to enhance patient treatment plans.
Contribute to Medical Literature: Publish studies that inform clinical guidelines and policies.
A decline in clinical researchers has raised concerns about the future of healthcare innovation, underscoring the need for more physicians to engage in research.
3. Nurses
Nurses play a pivotal role in patient care and are integral to the success of clinical trials. With clinical research training, nurses can:
Coordinate Clinical Trials: Manage day-to-day operations, including patient recruitment and data collection.
Educate Patients: Inform participants about study protocols, ensuring informed consent.
Monitor Patient Outcomes: Assess and document patient responses to interventions.
Clinical research nursing offers a rewarding career path, combining patient care with scientific inquiry.
4. Science Graduates
Individuals with degrees in life sciences, biotechnology, or related fields possess the analytical skills necessary for clinical research. Training provides them with:
Understanding of Clinical Trial Processes: Comprehend the phases and methodologies of trials.
Data Management Skills: Handle and analyze complex datasets effectively.
Regulatory Knowledge: Familiarity with guidelines governing clinical research.
Science graduates can pursue roles such as clinical research associates, data managers, or regulatory affairs specialists.
Benefits of Clinical Research Training
Enhanced Career Opportunities: Opens doors to diverse roles within pharmaceutical companies, contract research organizations, and academic institutions.
Contribution to Medical Advancements: Play a direct role in developing new therapies and improving patient outcomes.
Interdisciplinary Collaboration: Work alongside professionals from various fields, enriching professional experience.
Personal Fulfillment: Engage in work that has a tangible impact on public health.
Conclusion
Clinical research is crucial for advancing medical treatments and improving patient care. Training in this field equips professionals with the necessary skills to ensure the safety, efficacy, and ethical standards of clinical trials. As the demand for skilled researchers grows, investing in clinical research training is a smart choice for healthcare professionals. At CCRPS, we are committed to helping you build a successful career in clinical research as we move into 2025 and beyond.
Frequently Asked Questions (FAQs)
-
Clinical research involves studies conducted with human participants to evaluate medical, surgical, or behavioral interventions. It is crucial because it helps determine the safety and efficacy of new treatments, improving patient care and advancing medical knowledge.
-
Description text goes hereClinical research training is ideal for healthcare professionals such as pharmacists, doctors, nurses, and science graduates who are interested in expanding their skills in research, trial management, and regulatory compliance.
-
The length of clinical research training varies depending on the program. Basic certification programs can take a few weeks to months, while more advanced training and degree programs may take a year or longer.
-
Clinical trials typically follow four phases:
Phase I: Small groups test safety and dosage.
Phase II: Larger groups assess effectiveness.
Phase III: Larger groups confirm effectiveness and monitor side effects.
Phase IV: Post-approval studies to gather additional data on long-term effects.
-
Upon completing clinical research training, individuals can pursue careers such as Clinical Research Associate (CRA), Clinical Data Manager, Regulatory Affairs Specialist, Medical Writer, and Clinical Operations Manager, among others.