How to Improve Patient Retention in Clinical Trials: 5 Expert Tips
The success of clinical trials depends heavily on patient retention. The continuity of participant numbers throughout research studies protects data quality while fulfilling regulatory standards and producing meaningful results. The research outcomes of clinical trials remain at risk because patients frequently discontinue their participation despite the numerous obstacles clinical trials encounter to maintain patient engagement.
In this blog, we will delve into common reasons for patient dropout, explore effective retention strategies, and highlight the vital role of Clinical Research Coordinators (CRCs) in fostering patient engagement. By applying these expert tips, clinical trial teams can improve retention, ensuring trials are completed successfully and yield reliable data.
Common Reasons for Dropout in Clinical Trials
Understanding why patients leave a clinical trial is the first step in combating dropout rates. Many factors contribute to patient discontinuation, and they can vary based on the nature of the trial, the demographic of the participants, and the clinical setting. Here are some common reasons:
Side Effects of Treatment: Patients will drop out if they experience significant side effects from the treatment being tested. Poorly managed adverse effects that are associated with clinical trials can cause participants to drop out of the study.
Lack of Immediate Benefits: Patients who join clinical trials often seek prompt advantages from new treatments for chronic diseases during the research process. The trial becomes uninteresting to participants when they do not perceive any benefits from the research which leads them to withdraw from the study.
Time and Financial Burdens: Patients face significant challenges because the extensive time needed for visits and tests along with follow-ups becomes difficult to manage particularly when they need to travel far distances. The financial burden caused by trial expenses that exceed coverage or work absences leads patients to withdraw from the study.
Poor Communication: The failure to communicate between the trial team and participants can result in frustration and confusion. If patients do not feel that they are being informed about the study's progress or their role, they may decide to leave.
Unclear Expectations: The uncertainty about what participants are expected to do throughout the trial, including procedures, monitoring, and follow-up visits, can cause discomfort and uncertainty. It is therefore important to ensure that patients are well informed from the onset of what they are expected to do.
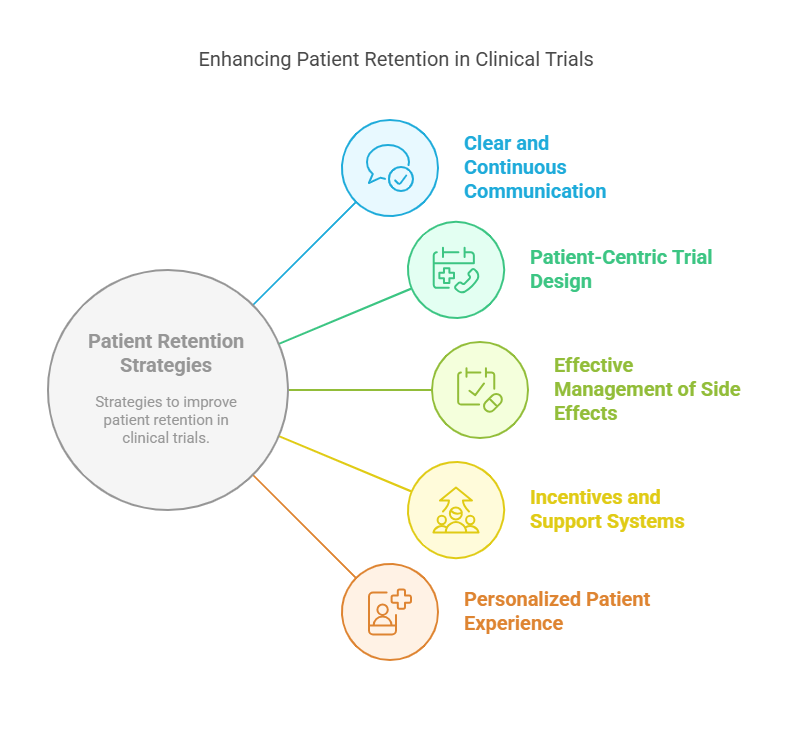
Effective Patient Retention Strategies
Improving patient retention in clinical trials requires proactive strategies that address these common reasons for dropout. Here are five expert tips for enhancing retention:
1. Clear and Continuous Communication
Clear and continuous communication is essential from the moment patients enroll in a clinical trial. CRCs should ensure that participants fully understand the trial’s purpose, process, and potential risks. Patients can stay engaged and feel valued when they receive regular updates about the trial’s progress.
Additionally, CRCs should maintain consistent follow-up communications to remind patients of appointments, address concerns, and update them on any changes in the protocol. This approach helps build a relationship of trust and reassures participants about their involvement.
2. Patient-Centric Trial Design
Study design should prioritize patient needs and circumstances as a method to enhance participant retention. The combination of flexible visit schedules with remote monitoring options and virtual consultations decreases substantial costs and time burdens for participants. Patients maintain their study participation when researchers minimize clinic visits to fewer sessions and schedule appointments at convenient times.
Moreover, providing travel reimbursements or compensating for lost wages can alleviate some of the financial pressures that might lead to dropout. Trial designs that accommodate the participants' lives are more likely to keep patients engaged throughout the study.
3. Effective Management of Side Effects
Patients are more likely to remain in a trial if they feel their side effects are being properly managed. CRCs should have a robust system in place to monitor and address side effects in real time. Proactive strategies include adjusting dosages, providing supportive treatments, and offering guidance on managing symptoms.
Having a dedicated medical team to monitor side effects and respond quickly to any adverse events can help reassure patients that their safety is a priority. Open lines of communication where patients can report issues immediately also play a crucial role in reducing dropout due to adverse effects.
4. Incentives and Support Systems
Incentives are a powerful motivator for patient retention. Offering monetary compensation, gift cards, or travel reimbursement for trial-related activities can encourage participants to remain in the study. Additionally, providing non-monetary incentives such as access to educational materials, personalized health reports, or recognition certificates can boost patient engagement.
Alongside incentives, building a support system around participants can also improve retention. Offering emotional support or creating patient groups to interact and share experiences can reduce feelings of isolation and make participants feel more connected to the trial process.
5. Personalized Patient Experience
Every patient is different, and tailoring the clinical trial experience to each participant's unique needs can significantly improve retention. Providing one-on-one consultations, personalized care plans, or customized follow-up schedules helps ensure that each patient feels understood and valued.
Ensuring patients feel they are not just a number but a vital part of the trial can lead to higher satisfaction and reduced dropout rates. This approach fosters trust between the participants and the clinical research team, ultimately increasing the likelihood of successful completion.
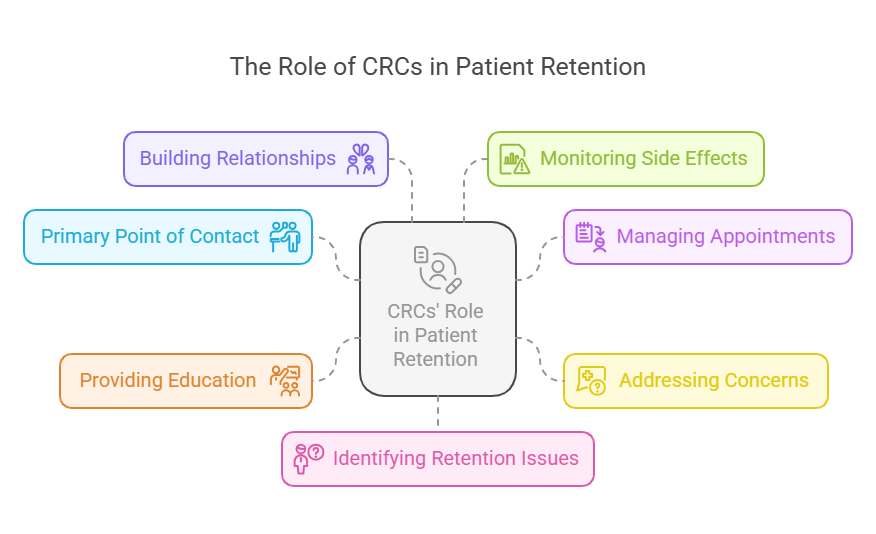
The Role of CRCs in Patient Retention
Clinical Research Coordinators (CRCs) are integral to the smooth functioning of clinical trials. They serve as the main point of contact for trial participants, ensuring that patients are well-supported and well-informed throughout the duration of the study. Their role is multifaceted and vital, especially when it comes to patient retention. Below is a thorough explanation of how CRCs contribute to keeping patients engaged and reducing dropout rates.
1. Primary Point of Contact for Participants
One of the primary responsibilities of CRCs is to act as the central liaison between the clinical trial team and the participants. As the first point of contact, CRCs are responsible for ensuring that patients understand the objectives of the trial, the procedures involved, and their specific role. They provide patients with all necessary information before they even begin the trial, helping them make an informed decision about their participation.
Once the trial begins, CRCs continue to communicate regularly with participants, addressing any concerns they may have and providing them with updates on the progress of the trial. This consistent communication helps build a rapport with patients, making them feel more connected to the study and its outcomes.
2. Managing Appointments and Scheduling
CRCs play an essential role in managing patient appointments, which can often be a logistical challenge in clinical trials. They are responsible for scheduling visits, arranging follow-ups, and ensuring that patients adhere to the trial protocol. This includes managing timelines for various assessments, tests, and treatments that need to be carried out at specific intervals during the trial.
By organizing these appointments efficiently, CRCs can minimize patient frustration and inconvenience, which might otherwise contribute to high dropout rates. They ensure that trial participants do not face unnecessary delays or conflicts in their schedules, making it easier for patients to stay on track throughout the study.
3. Addressing Concerns and Providing Support
Patients participating in clinical trials often have concerns regarding their health, the trial's progress, or the safety of the treatment they are receiving. CRCs are critical in addressing these concerns in a timely and empathetic manner. By being available to answer questions and provide reassurance, CRCs help mitigate anxieties that could lead to patient dropout.
Moreover, CRCs can identify potential barriers to continued participation early on. If a patient is struggling with side effects, scheduling conflicts, or a lack of understanding about the treatment, the CRC can step in to address these issues. By providing timely support, they can often prevent patients from deciding to leave the study prematurely.
4. Providing Education and Information
A key part of the CRC’s role is to educate participants about the clinical trial. In many cases, patients may be unfamiliar with clinical research, so providing them with clear, concise, and accurate information is vital for their continued participation. CRCs ensure that participants fully understand the trial’s protocols, what is expected of them, and the potential risks and benefits involved.
Ongoing education during the trial is equally important. If a patient feels they are not well-informed or if there is a lack of clarity regarding any aspect of the trial, they may become disengaged. CRCs keep patients informed about the progress of the study, what the next steps are, and how their involvement is contributing to the success of the trial. This fosters a sense of purpose and importance, helping patients remain committed to the study.
5. Building Strong Relationships with Participants
Building a strong relationship with participants is perhaps the most significant factor in patient retention. CRCs act as the face of the trial, and the relationship they develop with patients directly influences their willingness to stay enrolled. Patients who feel they have a personal connection with their CRC are more likely to continue participating in the trial.
A CRC who is friendly, approachable, and empathetic can create an environment where patients feel valued and respected. This emotional connection makes participants more invested in the success of the trial and more willing to endure the inconveniences or potential risks that may come with participation. It also increases trust, which is crucial when navigating the often challenging aspects of clinical trials, such as side effects or long study durations.
6. Monitoring and Reporting Side Effects
One of the biggest concerns for clinical trial participants is the potential for side effects from the treatment being tested. CRCs are responsible for monitoring patients for any adverse effects and reporting these to the medical team. By closely observing patients and documenting any side effects, CRCs help ensure the safety of participants and maintain the integrity of the trial data.
Proactive management of side effects is crucial for retention. When side effects occur, CRCs are responsible for addressing them promptly. Whether it’s offering advice on symptom management or providing additional resources, CRCs ensure that patients feel supported throughout the process. In some cases, they may work with the medical team to adjust the treatment protocol if necessary. This not only enhances the patient’s experience but also helps prevent patients from dropping out due to discomfort or fear of worsening symptoms.
7. Identifying and Mitigating Retention Issues
CRCs are trained to recognize signs that a patient might be considering dropping out of the trial. These signs can include missed appointments, lack of communication, or expressed frustration with the trial. By identifying potential retention issues early, CRCs can take steps to address them before they lead to dropout.
For example, if a patient is missing appointments due to travel difficulties, the CRC may offer virtual consultations or assist in arranging transportation. If a participant expresses concern about the trial’s impact on their lifestyle, the CRC can provide more flexible scheduling or offer additional support. By taking a proactive approach to retention, CRCs can resolve issues before they escalate, ensuring that patients remain committed to the trial.
8. Reporting Adverse Events and Adjusting Treatment Protocols
One of the most critical aspects of maintaining patient safety and retention in clinical trials is ensuring that adverse events are promptly reported and managed. CRCs are responsible for documenting and communicating any adverse events to the study's medical team and regulatory authorities. This helps ensure that patients are not put at unnecessary risk and that their health is continuously monitored.
In cases where side effects or adverse events are severe, CRCs may collaborate with the study physician to adjust the treatment protocol. This could include altering dosages, switching medications, or providing supportive treatments to mitigate the adverse effects. By making these adjustments, CRCs demonstrate a commitment to patient well-being, which can help retain participants who may otherwise feel compelled to drop out due to adverse reactions.
Lesser-Known Facts
Early Dropout Can Lead to Bias: High dropout rates early in the trial can skew the results, particularly in studies where patients who drop out are systematically different from those who remain. This can lead to attrition bias, affecting the validity of the study outcomes. (Source)
Electronic Monitoring Devices Help Retention: The use of digital health tools such as wearable devices can help reduce dropout rates by enabling remote monitoring and reducing the need for in-person visits. This approach enhances participant convenience and engagement. (Source)
Retaining Patients Saves Money: Trials with higher retention rates are more cost-effective because they avoid the need to recruit new participants. High dropout rates can lead to wasted resources and increased expenses, as recruiting and enrolling new participants is costly and time-consuming. (Source)
Explore Courses for Clinical Research Career
Courses Available:
Conclusion
Clinical Research Coordinators (CRCs) play a crucial role in enhancing patient retention in clinical trials. Their responsibilities, from managing communication and appointments to addressing concerns and monitoring side effects, directly impact the success of a study. By fostering strong relationships and creating a patient-centric environment, CRCs ensure that participants feel supported, ultimately reducing dropout rates and improving trial outcomes. For organizations like CCRPS, recognizing and empowering the role of CRCs is key to achieving successful and reliable clinical trials.
Frequently Asked Questions (FAQs)
-
The main reasons include adverse side effects, lack of immediate benefits, time and financial burdens, poor communication, and unclear expectations.
-
Improving retention involves strategies like clear communication, patient-centric trial designs, managing side effects effectively, providing incentives, and offering personalized support.
-
CRCs are responsible for maintaining communication with patients, addressing concerns, managing appointments, and creating a supportive environment, all of which help improve retention.
-
Incentives, such as financial compensation, gift cards, or recognition, can encourage patients to stay in clinical trials by reducing the time and financial burdens associated with participation.
-
Using electronic monitoring devices, partnering with patient advocacy groups, and offering remote or virtual trial options can all significantly improve patient retention rates.