Standard Operating Procedures (SOPs) in Clinical Research: A Quick Guide
Clinical research is an intricate process that requires a high degree of precision, consistency, and quality control to ensure patient safety and the integrity of the research data. One of the most crucial components of successful clinical trials is the development and implementation of Standard Operating Procedures (SOPs).
These are essential documents that outline the step-by-step instructions for conducting tasks in clinical research, ensuring that they are performed consistently and according to regulatory guidelines. This guide will delve into what SOPs are, their significance in clinical trials, and how to create effective SOPs that can drive efficiency and compliance in your clinical research processes.
What Are SOPs?
Standard Operating Procedures (SOPs) are detailed, written instructions that outline specific tasks and processes required in clinical research. SOPs are designed to ensure that all activities are conducted consistently and in compliance with regulatory standards. They serve as a reference for research teams, providing clear and standardized guidelines for performing tasks related to patient recruitment, data collection, informed consent, monitoring, and reporting. In essence, SOPs are the backbone of clinical trials, enabling researchers to follow a uniform approach to maintain the quality and integrity of their research efforts.
SOPs can cover a wide range of clinical research activities, such as:
Study initiation and protocol development
Informed consent procedures
Participant screening and recruitment
Data management and documentation
Monitoring and quality assurance
Adverse event reporting and handling
By defining roles, responsibilities, and actions in a systematic way, SOPs help prevent deviations, reduce errors, and increase the overall efficiency of clinical research processes.
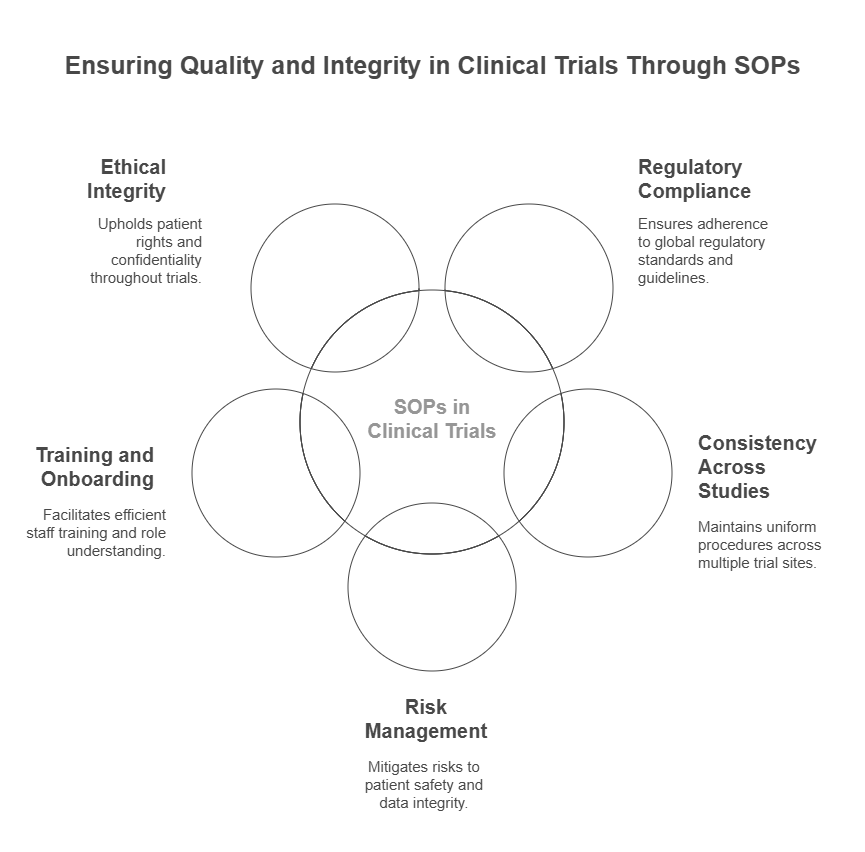
Importance of SOPs in Clinical Trials
SOPs are not just formalities — they are critical for maintaining the integrity and quality of clinical trials. Here are some reasons why SOPs are so important in clinical research:
1. Regulatory Compliance
SOPs help ensure that clinical trials are conducted in compliance with regulatory bodies such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and other global agencies. These organizations require strict adherence to established protocols and good clinical practice (GCP) guidelines. SOPs help clinical research organizations (CROs) and research sites meet these regulatory requirements.
2. Consistency Across Studies
Clinical trials often involve multiple sites, teams, and researchers. SOPs ensure that all team members follow the same procedures, leading to consistency across sites. This uniformity helps minimize variability in results and ensures that the data collected is reliable.
3. Risk Management
Having clear SOPs in place helps to mitigate risks, particularly in areas such as patient safety, data integrity, and study conduct. SOPs provide guidelines for handling adverse events, protocol deviations, and ethical concerns, which are crucial for managing risks in clinical research.
4. Training and Onboarding
SOPs are a key resource for training new staff members. They provide a step-by-step guide for performing clinical trial tasks, helping to ensure that all team members understand their roles and responsibilities. Well-documented SOPs simplify the onboarding process and reduce the time needed for new staff to become proficient.
5. Audit and Monitoring
During audits or inspections by regulatory bodies, SOPs act as a reference to demonstrate that clinical trials are being conducted according to approved processes. SOPs ensure that researchers have a standardized method for documenting procedures and monitoring activities, making it easier to demonstrate compliance during audits.
6. Improved Quality Control
SOPs help maintain high-quality standards in clinical research. By standardizing processes and ensuring they are followed precisely, SOPs reduce errors and improve the overall quality of the trial data. Consistent quality control is essential for ensuring that the trial results are valid and reproducible.
7. Ethical Integrity
SOPs ensure that ethical principles, such as patient confidentiality, informed consent, and transparency, are consistently upheld throughout the trial. SOPs guide researchers in how to handle sensitive information and ensure that patient rights are protected throughout the study.
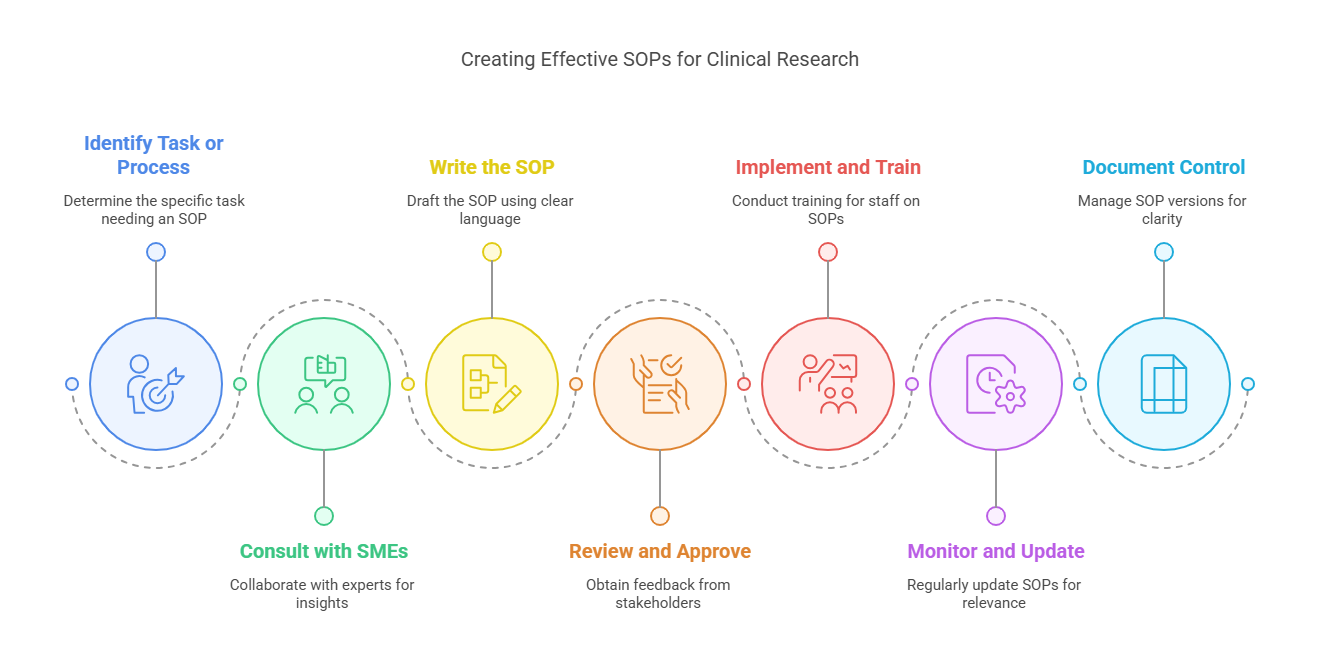
How to Create Effective SOPs?
Creating effective SOPs for clinical research requires careful planning, thorough documentation, and regular review. Here’s a step-by-step guide on how to create and maintain SOPs for your clinical research trials:
1. Identify the Task or Process
Start by identifying the specific task or process that requires an SOP. This could be anything from patient recruitment to data management or adverse event reporting. Clearly define the scope of the SOP and the purpose it will serve.
2. Consult with Subject Matter Experts (SMEs)
To ensure accuracy and relevance, collaborate with experts in the specific area of research. Subject matter experts can provide valuable insights into the best practices and regulatory requirements for the process you are documenting.
3. Write the SOP
When writing the SOP, use clear, concise language. Outline the procedure in a step-by-step format to make it easy for staff members to follow. Include details such as:
Roles and responsibilities
Equipment and materials needed
Detailed steps to complete the task
Safety and ethical considerations
Documentation and reporting requirements
Make sure the SOP is easy to understand for people with varying levels of expertise. It should be written in plain language and avoid jargon that may cause confusion.
4. Review and Approve
Before implementing the SOP, have it reviewed by relevant stakeholders, including clinical researchers, project managers, and regulatory experts. Their feedback will ensure that the SOP is accurate, comprehensive, and aligned with the research protocols.
5. Implement and Train
Once approved, implement the SOP and train all relevant staff on its use. Provide training sessions and workshops to ensure that everyone understands the procedures and knows how to apply them during the clinical trial.
6. Monitor and Update Regularly
Clinical research evolves over time, and so should your SOPs. Regularly review and update SOPs to reflect changes in regulations, new research technologies, or modifications to trial procedures. Keeping your SOPs up to date ensures that they remain relevant and effective.
7. Document Control
Establish a system for managing SOPs, including version control, so that the most current version is always used. This will help avoid confusion and ensure that all staff are using the most up-to-date guidelines.
Lesser-Known Facts About SOPs in Clinical Research
SOPs Reduce Human Error: Standardizing processes helps minimize errors, which is critical in clinical research where small mistakes can have significant consequences. SOPs ensure consistency and reduce variability in processes, thereby reducing errors. (Source)
Multiple SOPs for Different Phases: Clinical research involves different phases such as pre-study, study conduct, and post-study. Each phase may require its own set of SOPs to ensure compliance and consistency throughout the trial. (Source)
SOPs Support Quality Management Systems (QMS): Well-written SOPs are a cornerstone of a robust quality management system, which is vital for maintaining high standards in clinical trials. SOPs ensure that research is conducted consistently and efficiently. (Source)
Not All SOPs Are Regulatory Documents: While some SOPs are required by regulatory bodies, others are internal documents that aim to streamline operations and improve efficiency. These internal SOPs are not mandated by regulations but are essential for maintaining consistency. (Source)
Training Requirements for SOPs: Proper training in SOPs can significantly reduce protocol deviations and enhance compliance across all trial sites. Regular training and refresher courses are essential for maintaining SOP adherence. (Source)
Explore Courses for Clinical Research Career
Courses Available:
Conclusion
In conclusion, SOPs are essential for ensuring consistency, regulatory compliance, and data integrity in clinical research. They provide clear guidelines for research teams, helping to standardize processes and minimize errors. By regularly updating SOPs and involving key stakeholders in their creation, clinical research organizations can improve the efficiency and quality of their trials. As the clinical research landscape evolves in 2025, SOPs will remain vital in ensuring trials are conducted safely and effectively. CCRPS is dedicated to assisting organizations in developing SOPs that enhance the success of their clinical studies.
Frequently Asked Questions (FAQs)
-
SOPs ensure consistency, reduce human error, and help maintain regulatory compliance, all of which are crucial for the success of clinical trials.
-
SOPs should be reviewed and updated regularly, especially when there are changes in regulations, procedures, or technology in clinical research.
-
Subject matter experts, regulatory professionals, project managers, and clinical researchers should collaborate to create effective SOPs.
-
SOPs are detailed guidelines for conducting tasks, while protocols define the overall research design and objectives of a clinical trial.
-
Yes, SOPs can be standardized for recurring tasks across different trials, but may need to be customized depending on the specifics of each trial.