Top Benefits of Pursuing Clinical Research Training
Embarking on a career in clinical research offers numerous advantages, including career growth, skill development, global opportunities, and valuable certifications. As the healthcare landscape evolves, the demand for skilled clinical research professionals continues to rise, making this field both dynamic and rewarding.
Career Growth in Clinical Research
The clinical research industry is growing rapidly and globally. This is mainly because pharmaceutical companies, biotech firms, and government health departments are heavily investing in research and development (R&D). New diseases, evolving healthcare needs, and the demand for personalized medicine all require extensive clinical trials.
According to the U.S. Bureau of Labor Statistics, jobs in medical and health services management (which includes many clinical research roles) are expected to grow by 18% between 2022 and 2032, which is much faster than average for other fields. This means more job openings, career options, and higher salaries in the coming years.
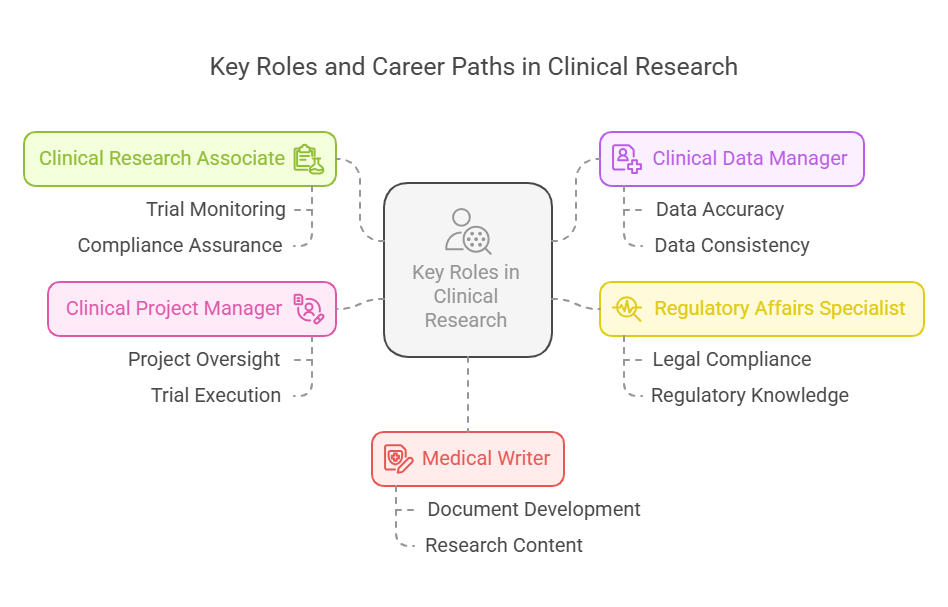
Key Roles and Career Paths:
Clinical Research Associate (CRA) – Monitors clinical trials, ensuring they follow regulatory and ethical standards.
Clinical Data Manager – Handles trial data to ensure accuracy, consistency, and quality.
Regulatory Affairs Specialist – Manages the legal and regulatory side, helping companies comply with national and international laws.
Clinical Project Manager – Oversees the execution of trials from start to finish.
Medical Writer – Develops clinical documents and research-based content.
Each of these roles allows you to climb the ladder with experience. For example, a CRA can advance to senior CRA, project manager, or clinical operations director with the right qualifications and experience.
Skill Development Through Clinical Research Training
Training programs in clinical research offer structured, in-depth education that prepares individuals for the real-world demands of the industry. These programs are not just about learning terminology — they involve case studies, regulations, real trial simulations, and even soft skills like communication and problem-solving.
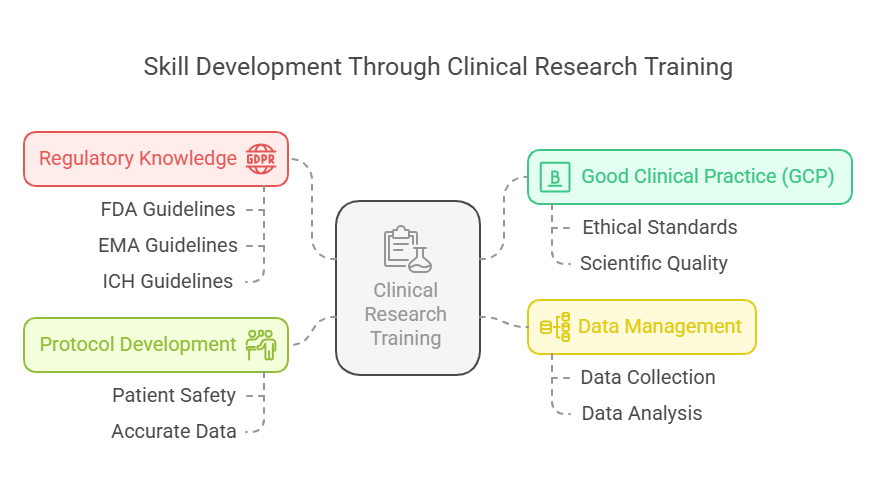
Key Skills Acquired:
Good Clinical Practice (GCP) – Global ethical and scientific quality standards for designing, conducting, and reporting trials.
Regulatory Knowledge – Understanding of FDA, EMA, ICH guidelines, and other regulatory bodies.
Data Management – How to collect, clean, and analyze data efficiently and securely.
Protocol Development – Designing research protocols that ensure patient safety and accurate data.
These skills are essential for getting hired and staying relevant in the fast-changing clinical research industry.
Global Opportunities in Clinical Research
Clinical trials happen all over the world, not just in the U.S. or Europe. Countries like India, China, South Africa, Brazil, and Australia are now major hubs for trials due to their diverse populations and cost-effective infrastructure.
This opens up global job opportunities in:
Multinational pharmaceutical companies
International Contract Research Organizations (CROs)
Regulatory bodies like the EMA or WHO
Global health nonprofits
Perks of Working Globally:
Exposure to multicultural work environments
Opportunities for travel or relocation
Better salary packages and experience
Broader scientific and medical knowledge
Clinical research professionals with international experience are often highly valued because they understand diverse regulations, ethics, and patient populations.
Importance of Certifications in Clinical Research
Certifications add credibility and professionalism to your profile. While not always mandatory, they are often a preferred or required qualification in job listings for roles such as CRA, regulatory affairs, or QA specialist.
Top Clinical Research Certifications:
Certified Clinical Research Professional (CCRP) – Offered by the Society of Clinical Research Associates (SoCRA), this is one of the most respected certifications in the field.
Certified Clinical Research Associate (CCRA) – Offered by the Association of Clinical Research Professionals (ACRP), it validates experience and competence in trial monitoring.
GCP Certification – Often available through online modules or short courses, proving your understanding of Good Clinical Practice.
ICH-GCP Certification – International Council for Harmonisation's globally accepted research standards.
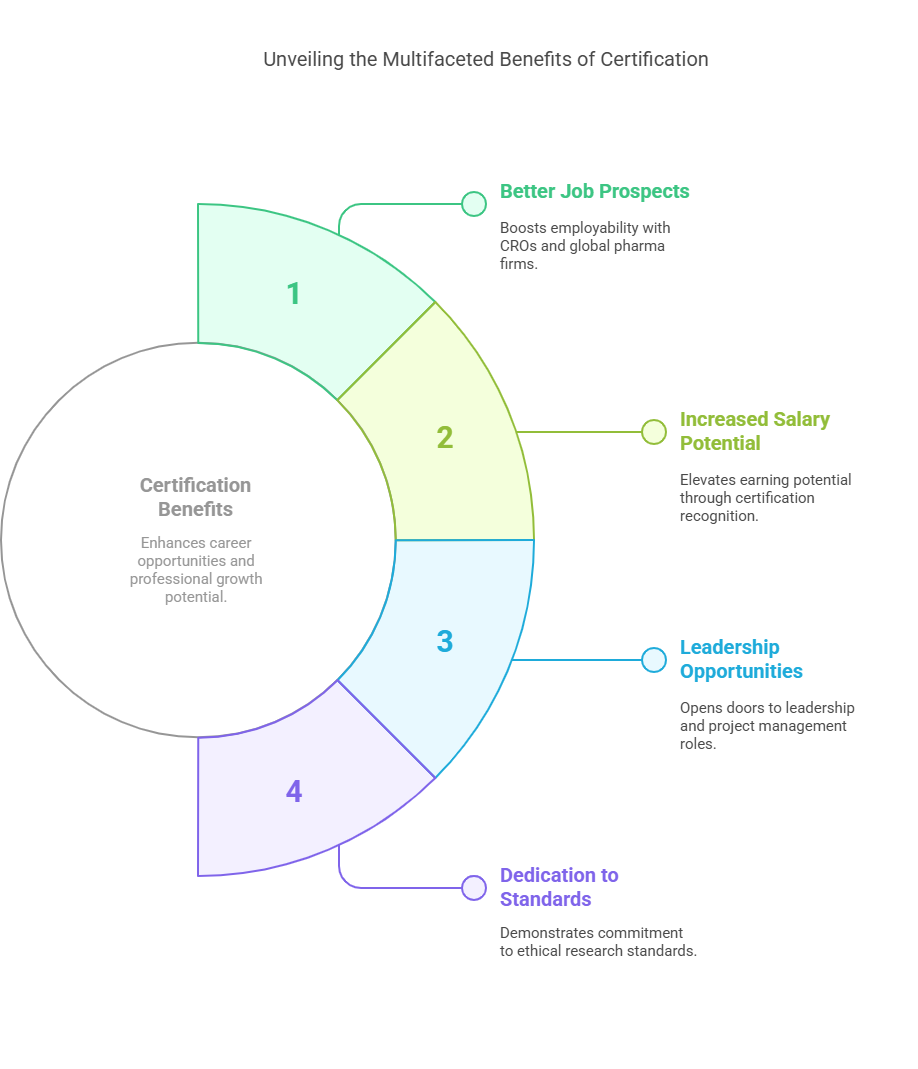
Benefits of Certification:
Better job prospects (especially with CROs or global pharma firms)
Increased salary potential
Opportunities for leadership and project management roles
Shows your dedication to maintaining ethical and compliant research standards
Certifications often require continuing education credits, which help professionals stay updated with the latest regulations and practices.
Lesser-Known Facts About Clinical Research
The first controlled clinical trial was conducted in 1747 by James Lind, who sought to find a cure for scurvy among sailors. Lind divided sailors into groups and tested different treatments, ultimately discovering the effectiveness of citrus fruits in combating scurvy. (Source)
The concept of a placebo dates back to the early 1800s, with its first documented use in medical literature as a "dummy remedy." Placebos are now essential in clinical trials for comparing the efficacy of new treatments. (Source)
Ensuring diverse participant representation is crucial for developing treatments effective across different populations. Without diversity, clinical trials risk producing results that may not be universally applicable. (Source)
Clinical trials are strictly regulated by agencies like the FDA to ensure participant safety and data integrity. Ethical guidelines such as the Nuremberg Code and Declaration of Helsinki have shaped modern clinical trial practices. (Source)
Explore Courses for Clinical Research Career
Courses Available:
Conclusion
Pursuing clinical research training offers a pathway to a fulfilling career characterized by continuous learning, global engagement, and the opportunity to contribute to medical advancements. By obtaining relevant certifications and staying abreast of industry trends, professionals can position themselves at the forefront of this vital field. Organizations like CCRPS provide comprehensive training programs to equip aspiring clinical researchers with the skills and knowledge necessary for success.
Frequently Asked Questions (FAQs)
-
Clinical research training is a structured program that teaches individuals how to plan, conduct, and monitor clinical trials while adhering to ethical and regulatory standards. It covers topics like Good Clinical Practice (GCP), trial design, data management, and regulatory compliance.
It is ideal for:
Medical graduates
Life sciences professionals
Nurses
Pharmacists
Allied health professionals
Anyone interested in a research-oriented healthcare career
-
No, a medical degree is not mandatory. Many successful professionals come from backgrounds in life sciences, pharmacy, nursing, biotechnology, and public health. Some clinical research roles, such as clinical data manager or regulatory associate, do not require direct patient interaction.
However, roles involving clinical assessments may prefer or require a medical or paramedical background.
-
The duration varies depending on the program and provider:
Short-term certifications: 1 to 6 months (ideal for freshers or career switchers)
Advanced diplomas or postgraduate programs: 6 to 12 months
Master’s degree programs: 1 to 2 years
-
After completing clinical research training, you can apply for various entry-level to mid-level roles, including:
Clinical Research Coordinator (CRC)
Clinical Research Associate (CRA)
Clinical Trial Assistant (CTA)
Data Manager
Regulatory Affairs Associate
Pharmacovigilance Officer
Quality Assurance Auditor
As you gain experience, you can move into roles like Clinical Project Manager, Director of Clinical Operations, or Medical Affairs Manager.