Clinical Research vs. Medical Research: Key Differences
The terms clinical research and medical research are often used interchangeably. However, while closely related, they represent different aspects of the healthcare research ecosystem. Understanding how they differ in terms of training, job roles, objectives, and applications is crucial for professionals pursuing careers in health sciences and for organizations involved in drug development, policy-making, or patient care.
In this blog, we’ll break down each field, explore how they diverge, highlight current trends as of 2025, and share lesser-known insights supported by credible references.
What Is Medical Research?
Medical research is a broad term that encompasses both basic and applied scientific studies aimed at advancing our understanding of human health and disease. It typically happens in labs, universities, research institutes, or pharmaceutical companies, and its ultimate goal is to uncover new knowledge that can improve healthcare delivery and outcomes.
Subtypes of Medical Research:
Basic (Pre-clinical) Research:
Involves animal studies, cellular analysis, and molecular biology techniques to understand diseases at the biological level.Translational Research:
Bridges the gap between basic science and patient care, turning discoveries from the lab into usable therapies.Epidemiological Research:
Studies disease patterns in populations to identify causes, trends, and risk factors.Health Services Research:
Evaluates the effectiveness, efficiency, and equity of healthcare services and delivery models.
What Is Clinical Research?
Clinical research is a subset of medical research that specifically involves human participants. Its goal is to determine the safety and efficacy of new drugs, medical devices, treatment protocols, and diagnostic methods.
Types of Clinical Research:
Clinical Trials (Phases I–IV):
Investigate new drugs or therapies in people, from safety testing to post-market surveillance.Observational Studies:
Monitor patient outcomes without altering their treatment.Behavioral Research:
Looks at how behavior affects health and how interventions might help.
Key Differences: Training, Roles, and Focus
While both fields contribute to the development of healthcare interventions, the required training, roles, and objectives diverge significantly.
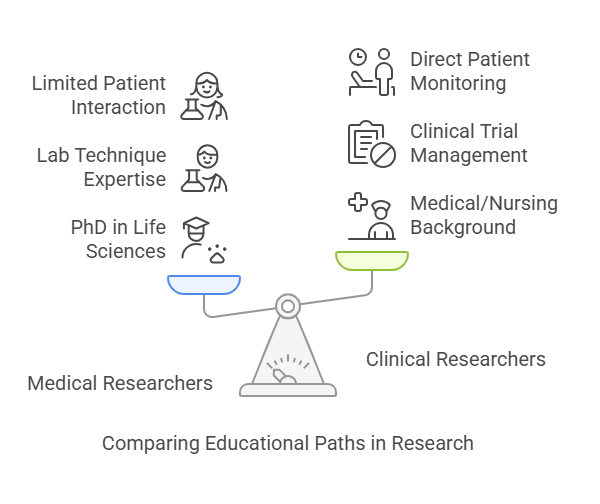
1. Educational Training
Medical Researchers:
Typically hold PhDs in life sciences (e.g., immunology, pharmacology).
Deep knowledge in lab techniques, hypothesis design, and scientific publishing.
Limited direct interaction with patients.
Clinical Researchers:
Backgrounds in medicine, nursing, pharmacy, or public health.
Often hold certifications like CCRP, ACRP, or GCP (Good Clinical Practice).
Trained in clinical trial management, ethical regulations, and patient monitoring.
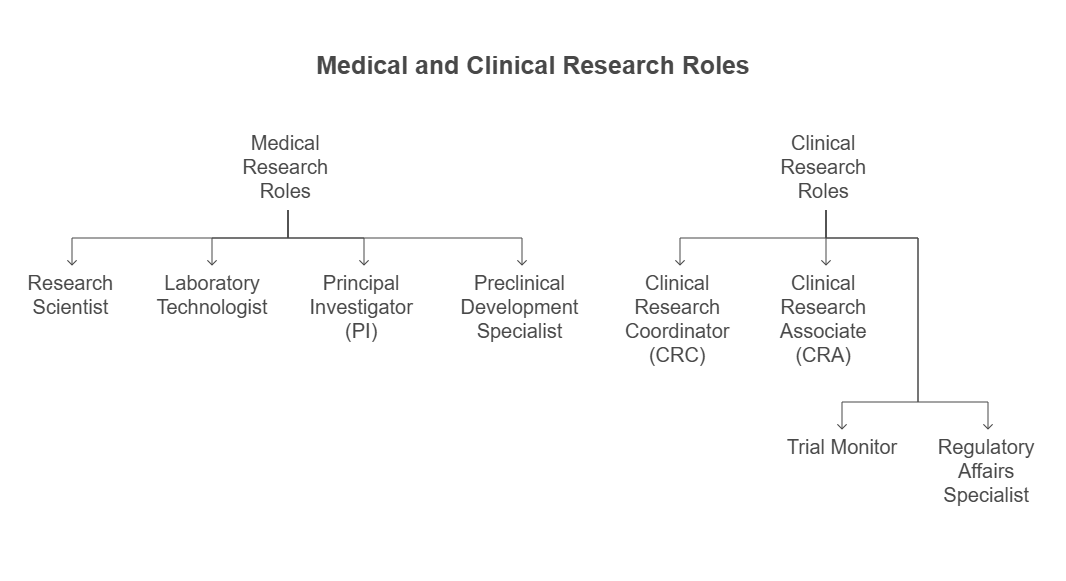
2. Job Roles
Medical Research Roles:
Research Scientist
Laboratory Technologist
Principal Investigator (PI)
Preclinical Development Specialist
Clinical Research Roles:
Clinical Research Coordinator (CRC)
Clinical Research Associate (CRA)
Trial Monitor
Regulatory Affairs Specialist
How Are These Fields Interconnected?
Despite their differences, clinical and medical research are interdependent. Breakthroughs from basic medical research often lead to new clinical trials. Conversely, clinical outcomes can prompt new lab investigations to explore mechanisms further.
For example, the discovery of mRNA technology (basic research) laid the foundation for the Pfizer and Moderna COVID-19 vaccines, which were validated through rigorous clinical trials.
Lesser-Known Facts
Medical research doesn’t require human involvement until clinical translation begins: Basic research focuses on understanding molecular, genetic, or cellular mechanisms of diseases without involving human participants. Human involvement typically begins during clinical translation, such as in preclinical trials and beyond. (Source)
Clinical trials can now use virtual reality to simulate patient experiences: Virtual reality (VR) is being used to enhance patient compliance by simulating treatment effects and improving patient understanding of their conditions and therapies. (Source)
Lab-grown organs are used in medical research to reduce animal testing: Organoids, such as lab-grown lungs, replicate human organ functions and are increasingly used to test drug efficacy and toxicity, reducing reliance on animal models. (Source)
More than 80% of trials in low- and middle-income countries lack proper ethics oversight: Many clinical trials conducted in these regions do not go through institutional review boards or ethics committees, raising concerns about exploitation and ethical standards. (Source)
Conclusion
While both clinical and medical research strive to advance human health, they operate in different spaces—one in the lab, the other at the patient’s bedside. Their synergy powers innovation, translating molecular discoveries into life-saving therapies. Whether you’re a student, a researcher, or a healthcare organization, understanding these differences can help you navigate and contribute meaningfully to this dynamic field.
If you're interested in building a career in clinical research, obtaining certified training through trusted institutions is key. At CCRPS, we offer internationally accredited courses in clinical trial management, monitoring, and research ethics—empowering professionals to lead in global healthcare innovation.
Frequently Asked Questions (FAQs)
-
Clinical research involves human participants, while medical research may involve cell cultures, animals, or simulations without direct patient interaction.
-
Yes. With appropriate training and cross-disciplinary experience, professionals can bridge both worlds—especially in translational medicine roles.
-
Not always. Many clinical research professionals hold medical or health-related degrees and certifications like CCRP.
-
No. They can test devices, lifestyle interventions, surgical techniques, and even health education tools.