Virtual Clinical Trial
Clinical Research Remote Summary
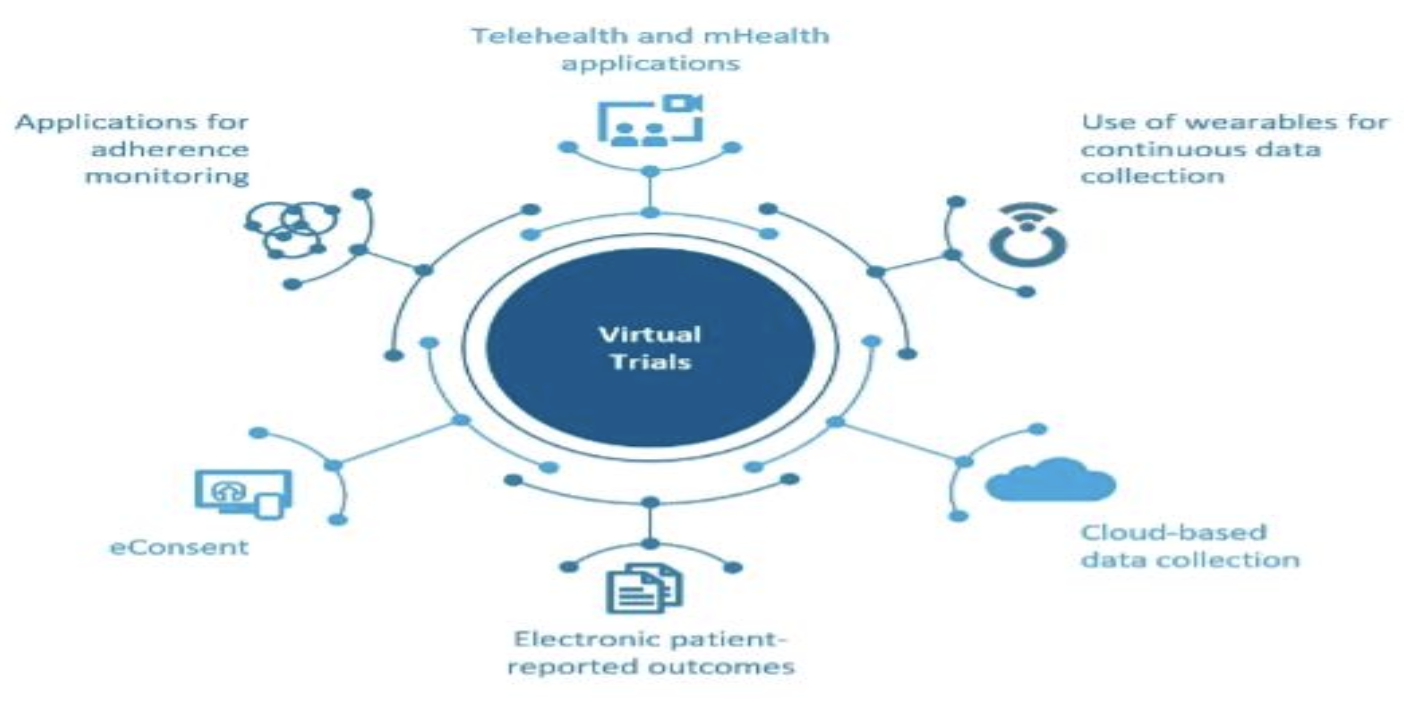
Fig 1.1 Virtual clinical trials
Over the years, conventional clinical trials, which have been a major part of medical advancement, have generally required participants to visit research sites for check-ups and data collection. But what if it was possible to be a part of great research while comfortable at home? Thus, the virtual clinical trials are shaping the innovation of medications and treatments in the USA in a completely new way.
What are Virtual Clinical Trials?
Through the use of technology, virtual clinical trials also referred to as decentralized trials or remote trials, conduct research remotely. Using web portals, mobile apps, wearable devices and telemedicine, participants complete study visits and share data electronically. This innovative approach breaks down geographical barriers and participants can access the study from the comfort of their homes than ever before. For those coordinating these trials, the Clinical Research Coordinator course is an excellent resource.
Benefits of Virtual Clinical Trials in the USA
Convenience: Take part in research from the comfort of your home, without having to deal with travel burdens or time constraints.
Increased Accessibility: For instance, use social media and community resources to reach out to more participants especially those in rural areas or with mobility challenges.
Real-World Data: Quantifying participation in a participant's natural setting offers a real-life view of treatment efficacy.
Improved Engagement: Enable participants to access and control their health information, and remain compliant with the research. For those who want to find out more, or are thinking of entering this profession, the Clinical Trials Assistant Training course is also worth checking out.
A New Era of Data Collection
Virtual clinical trials are a method of collecting safety and efficacy data throughout the entire research process from study start up to execution using technology and online social engagement platforms to prioritize patient comfort at every stage. For professionals wishing to build on their expertise in this innovative method, the Advanced Clinical Research Project Manager Certification might be worth considering.
The Impact of COVID-19
The COVID-19 pandemic has suddenly raised the challenge of remote clinical research methods on the pharmaceutical industry. Previusly, the industry had not undergone much practice with this approach, but the pandemic revealed great advantage of the virtual trials. Therefore, the industry is increasingly adopting this operational paradigm and the industry is moving fast in this regard. The oversights in trials may find the Medical Monitor Certification useful and the CRA (Clinical Research Associate) training may be helpful for the research conducters.
Remote Data Collection: Advantages and Considerations
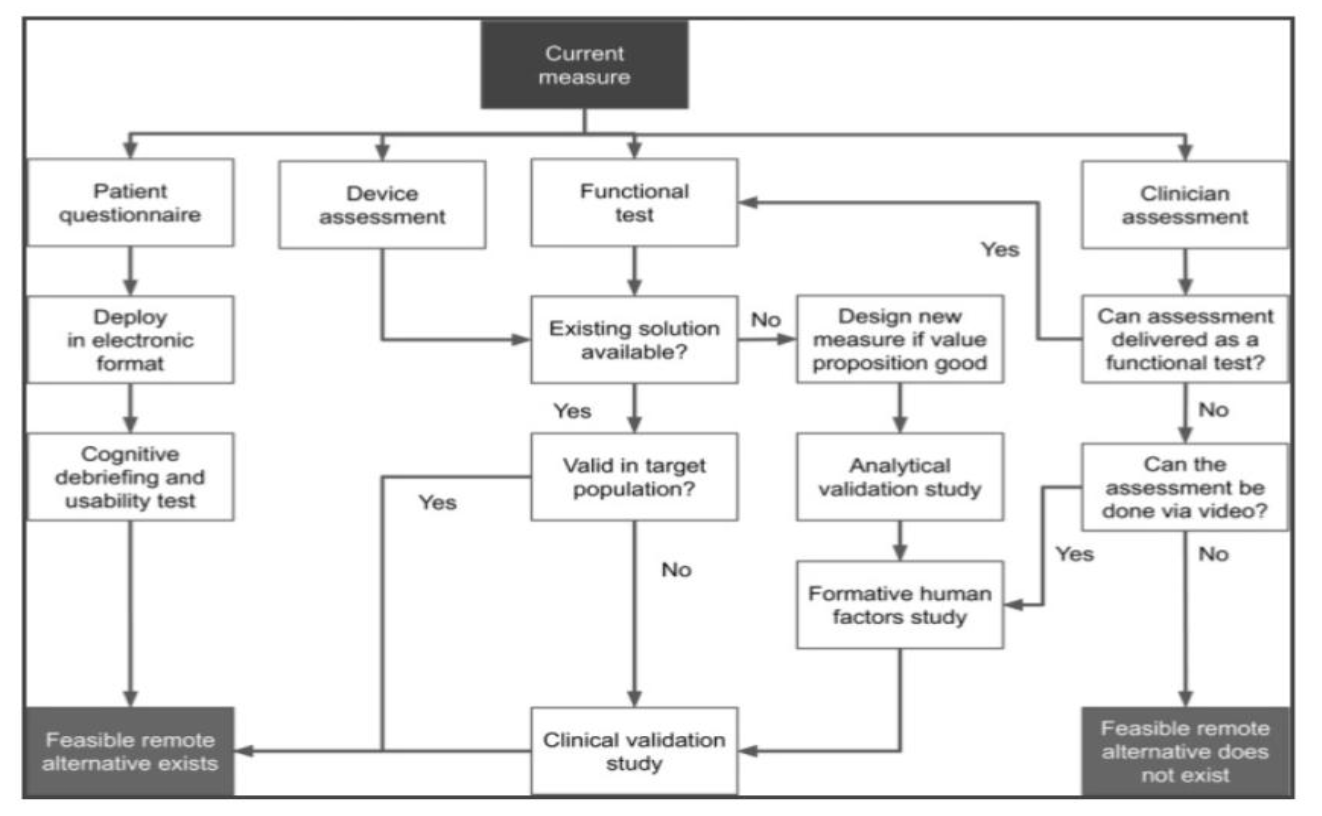
It should also be noted that remote data collection is not feasible for all types of measurements. To this end, researchers will have to develop a decision tree to determine which clinical measures can be effectively translated into reliable remote measurements.
Although it is feasible to conduct remote data collection, there are several crucial tasks that need to be performed in order to make sure that the data collected is safe, usable and valid. These activities entail establishing a measurement frequency, scheduling assessments for remote measurements, and dealing with the fact that many connected devices sample data continuously. For those interested in further focusing on data integrity and safety in remote trials, the Pharmacovigilance Certification offers extensive training. Furthermore, for physicians who want to become principal investigators in such innovative trials, the Advanced Principal Investigator Physician Certification is intended to provide them with extensive training.
The Balancing Act: Remote vs. Traditional Assessments
Collecting clinical trial data remotely presents both advantages and disadvantages. While it offers increased convenience and accessibility, it may not be suitable for all types of measurements. Recognizing these limitations and implementing robust data collection practices are essential for ensuring the success of virtual clinical trials.
Is a Virtual Clinical Trial Right for You?
If you are interested in helping advance medical knowledge and possibly gain access to new treatments, then virtual clinical trials may be ideal for you. But it’s important to realize that eligibility needs can differ based on the study.
Finding Virtual Clinical Trials in the USA
Numerous resources can help you find virtual clinical trials in the USA:
ClinicalTrials.gov: A comprehensive database of federally funded clinical trials including virtual ones.
Patient advocacy groups: Many organizations are helping patients to get connected with research studies that are related to their health condition.
Telehealth platforms: Some platforms- connect patients- with virtual trials- focused on various health areas.
The Future of Virtual Clinical Trials
As technology continues to evolve, virtual clinical trials are poised to play an even greater role in shaping the future of healthcare research in the USA. They offer a more efficient, inclusive, and patient-centric approach to developing life-saving treatments.
Fig 1.2 Decision tree for identification and implementation of remote measurement
FDA guidance on remote monitoring
FDA plays a crucial role in protecting the united states from threats such as emerging infections (including pandemics like coronavirus) e.g. FDA is issuing this guidance to help sponsors to some extent in order to assure the safety of participants, compliance and to minimize the risk to trial integrity. FDA guidance on the conduct of the clinical trial of the medical product during the COVID-19 pandemic is useful, for instance, to consider the safety of the drug or the correct use.
The progression of using a decentralized clinical trial model and remote data collection was limited before the COVID-19 pandemic. But then, the rapid adoption of telehealth during COVID-19 when remote doctor visits became vital. Thus, the emergence of remote monitoring clinical trials during COVID-19. The rate of adoption to remote measurements and the sharing of the experience and results could accelerate the field of clinical trials. During the COVID-19 pandemic, many details are still unknown regarding remote monitoring; nevertheless, it could be a good time to revamp the conventional clinical trial models.
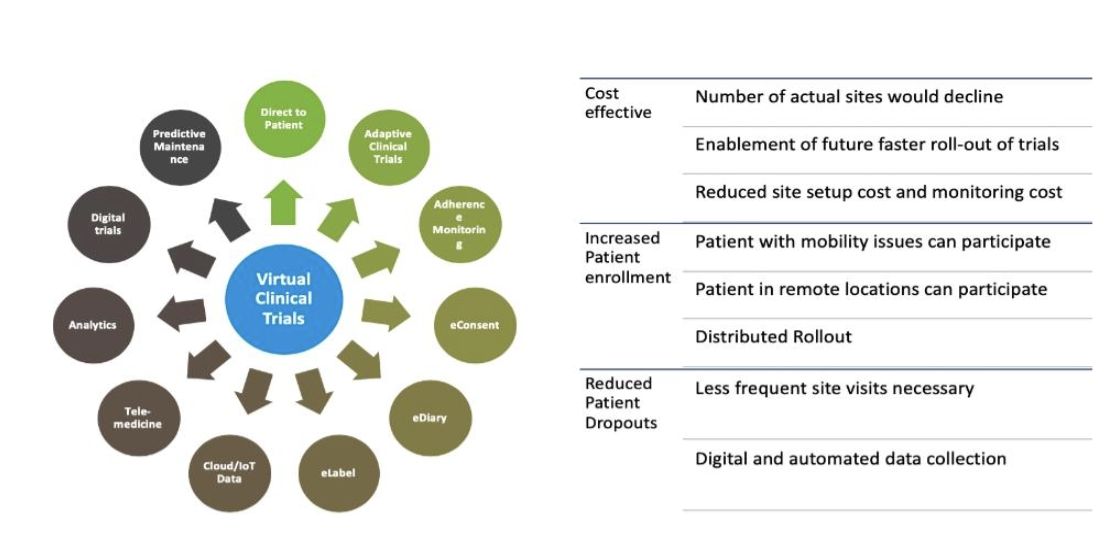
Through the use of distributed technologies vigorous clinical trials can be conducted through virtual clinical trials. Moreover, virtual clinical trials can cut the cost of the trial, decrease the time of the trial, enhance the rate of protocol adherence and improve the diversity of the trial members and participants. In this manner, the participant has access to the research team through a technology web portal. However, there is room for improvement regarding the collection of a lot of data. This approach has a growth potential and is also crucial in the social component of the clinical trials as well as the trust that can be developed between the participants and the researchers. A VR based communication hub for VCT should bring back some of the human elements to these studies. The use of VR in the relationship between the participant and the researchers is important in the single-blind study in supporting the relationship.
Fig 1.3 Virtual clinical trial monitoring
Explore Courses for Clinical Research Career
Courses Available:
Conclusion
In conclusion, virtual clinical trials are revolutionizing medical research by making it more accessible and efficient. As the field continues to evolve, resources like the Clinical Research Coordinator Professional Society (CCRPS) provide essential training and certification to professionals, ensuring that they are equipped to manage these innovative trials effectively.
References:
https://www.fda.gov/media/137496/download- FDA Guidance on Conduct of Clinical Trials of Medical Products During the COVID-19 Public Health Emergency
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7307062/ - Remote Monitoring in Clinical Trials During the COVID‐19 Pandemic
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7487205/ - Electronic consenting for conducting research remotely: A review of current practice and key recommendations for using e-consenting
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6969384/ - A Virtual Home for the Virtual Clinical Trial
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7051945/ - Conducting a Virtual Clinical Trial in HER2-Negative Breast Cancer Using a Quantitative Systems Pharmacology Model With an Epigenetic Modulator and Immune Checkpoint Inhibitors
Frequently Asked Questions (FAQs)
-
Participants in virtual clinical trials can enjoy greater convenience by engaging in research from their homes, eliminating the need to travel. This accessibility allows a broader and more diverse group of individuals to contribute to medical research. Additionally, virtual trials often use real-time data collection, providing a more accurate representation of how treatments work in daily settings.
-
Virtual clinical trials maintain safety and data accuracy through advanced technologies such as secure web portals and mobile apps that ensure data encryption and compliance with regulatory standards. Regular virtual check-ins, remote monitoring by healthcare professionals, and the use of validated measurement devices also play crucial roles in safeguarding participant data.
-
Eligibility for virtual clinical trials depends on the specific study's requirements, which can include age, medical history, current health status, and the condition being studied. Potential participants must meet these criteria to ensure the data collected will help achieve the research objectives.