What is the Difference Between a CRC and a Clinical Research Associate?
Getting into clinical research, the jobs of Clinical Research Coordinators (CRC) and Clinical Research Associates (CRA) are some of the most common. Both are important in the clinical trial process but the responsibilities, day to day tasks and the scope of the work are quite different. Knowing these differences will help people make the right decision about their career, and for those who are interested in clinical research, it will be useful to understand how these roles work together to guarantee the correctness and success of clinical trials. In this blog, we will try to distinguish between a CRC and a CRA, and how they contribute to the improvement of medical research.
The Role of a Clinical Research Coordinator (CRC)
A Clinical Research Coordinator (CRC) is usually found at the research site (e.g. hospitals, academic institutions, private clinics) and reports to the Principal Investigator (PI). The main role of the CRC is to oversee and administer the operational aspects of a clinical trial at the research site.
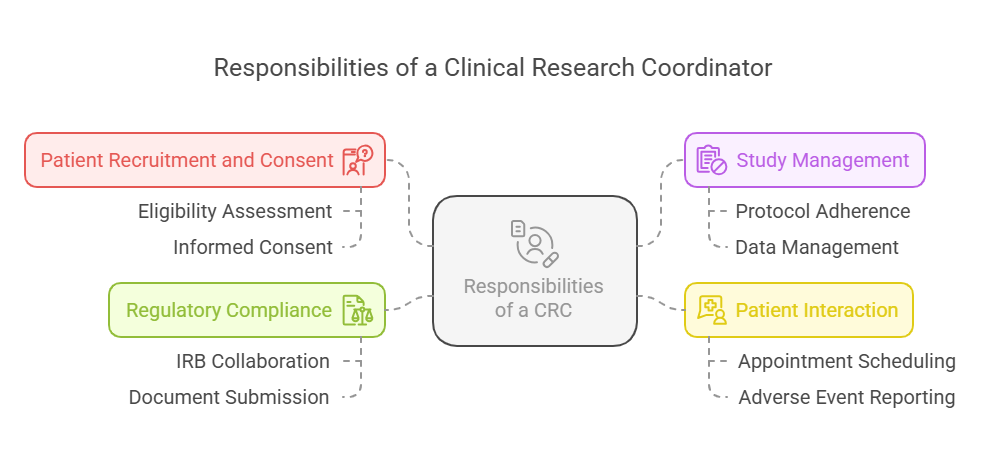
Responsibilities of a CRC
Patient Recruitment and Consent: One of the main duties of a CRC is participating in the enrollment of subjects in the clinical trial. This involves checking if the potential candidates are fit to be included in the study as defined by the study protocol. After eligible participants are spotted, the CRC gets the participant’s consent, which includes the risks, benefits, and why the study is being done.
Study Management: CRCs guarantee that the clinical trial is conducted in accordance with the study protocol, Good Clinical Practice (GCP) guidelines and, if relevant, any regulations. They keep very good records, data are collected and entered, and participants' compliance is monitored during the course of the study.
Patient Interaction: The CRCs are the primary contact with the participants. They make appointments, remind of the treatments, and notify the PI of any adverse events. The role can involve building good participation communication, so participants know what is happening in the study and what is expected of them.
Regulatory Compliance: CRCs also ensure that the clinical trial complies with the ethical guidelines and regulatory standards by working closely with the institutional review boards (IRBs) and regulatory authorities. They also help to prepare and submit the necessary documents to make the trial legal and ethical.
Skills Required for a CRC
Strong organizational abilities to manage various aspects of the trial simultaneously
Excellent communication and interpersonal skills to interact with participants, healthcare professionals, and study sponsors
Attention to detail to ensure data accuracy and protocol compliance
Problem-solving capabilities to address any issues that may arise during the trial
The Role of a Clinical Research Associate (CRA)
On the other hand, a Clinical Research Associate (CRA) is employed by a sponsor or contract research organization (CRO) to oversee clinical trials to ensure they are conducted properly as per the study protocol and regulatory requirements. CRAs are pivotal in overseeing various clinical sites, which often require them to travel between sites to ensure the consistency of trials across sites.
Responsibilities of a CRA
Site Monitoring: The CRAs are to ensure that the clinical trial sites are visited regularly in order to check on compliance with study protocols and regulatory requirements. Routine monitoring visits are made, data accuracy checked for and, operations at the site assessed. That is all very well, but when discrepancies are found they have to be resolved in order to maintain the integrity of the study.
Quality Control and Auditing: The CRAs review the collected data, complete it if it is missing, and make sure that it is accurate and matches the source documents (such as patient medical records). They check for adherence to the study protocol and GCP guidelines, and that the data reported to the sponsor is of good quality and trustworthy.
Training Site Personnel: In addition, CRAs train site staff on study procedures and protocols to prevent wrong data collection and bad trial conduct. They may also be able to assist with guidance on regulatory submissions and compliance with international guidelines.
Problem Solving: CRAs identify problems at the clinical sites and help the site personnel to fix them. They could be as small as problems with data collection or as big as failure to comply with the study protocol.
Skills Required for a CRA
Strong analytical abilities to assess data accuracy and protocol adherence
Flexibility and willingness to travel frequently, as the job often requires on-site visits
Excellent communication skills to collaborate with site staff and other stakeholders
An understanding of regulatory guidelines and clinical trial processes to ensure compliance
Key Differences Between a CRC and a CRA
While both CRCs and CRAs are integral to the clinical trial process, their roles are distinct in several key areas:
1. Location of Work
CRC: Based at the research site, working closely with participants and site staff.
CRA: Frequently travels between clinical sites, often working remotely or at a sponsor's office.
2. Day-to-Day Responsibilities
CRC: Manages daily trial operations at the site, focusing on participant recruitment, interaction, and data collection.
CRA: Monitors multiple sites for compliance, ensuring that the data collected is accurate and that the trial is being conducted correctly.
3. Point of Contact
CRC: Directly interacts with trial participants and is their primary point of contact throughout the study.
CRA: Primarily interacts with site personnel and the sponsor, providing oversight rather than direct patient interaction.
4. Scope of Oversight
CRC: Works on one or more trials at a single site, focusing on the internal operations of those specific studies.
CRA: Oversees multiple sites or trials, ensuring consistency and compliance across all of them.
5. Reporting Structure
CRC: Reports to the Principal Investigator (PI) at the research site.
CRA: Reports to the sponsor or CRO, acting as a liaison between the site and the sponsor.
Collaborative Roles
CRCs and CRAs are often different in their role and responsibilities. The CRA depends on the CRC to supply accurate information and to make sure the trial is proceeding without a problem at the site. However, the CRC depends on the CRA for oversight and to resolve any of the regulatory or compliance issues that may occur. This collaboration is necessary to ensure the proper conduct of the trial and the accuracy of the data collected for the regulatory submission.
Career Path Considerations
Both roles are good career paths in clinical research but are designed for those with different preferences and skill sets. If you like working directly with patients and running operations at a single site, CRC might be the right career for you. However, if you like the look of looking over several trials, traveling, and making sure that everything is okay across many sites, then the CRA position might be more what you’re looking for.
Educational Requirements for CRCs and CRAs
Both CRCs and CRAs generally require a background in health sciences, life sciences, or a related field. Most positions require at least a bachelor's degree, though some may require further certifications or training.
The CRCs usually seek to obtain certifications like the ones provided by the Advanced Clinical Research Project Manager Certification or Clinical Research Coordinator training programs to increase their expertise.
CEs can also benefit from certification, such as through the Clinical Trials Assistant Training programs, which provide specialized training for those working at clinical sites.
Growth Opportunities and Industry Demand
Both CRCs and CRAs are in high demand due to the expanding global clinical research industry. The increasing complexity of clinical trials, coupled with the need for precise data collection and regulatory compliance, has amplified the need for qualified professionals in both roles.
According to recent industry reports, the global clinical trial market is expected to grow significantly over the next decade, further increasing the demand for trained CRCs and CRAs to support various phases of drug development and testing .
Choosing Between CRC and CRA Roles
Deciding between a career as a CRC or CRA ultimately depends on your personal preferences, professional goals, and desired lifestyle. CRCs typically have more stable, location-based work, which may appeal to those who prefer routine and direct patient interaction. CRAs, with their frequent travel and broader oversight responsibilities, may appeal to those seeking a more dynamic, varied work environment.
For those interested in both paths, some professionals start as CRCs before transitioning to CRA roles, allowing them to gain experience in clinical research management before moving into broader oversight positions.
The Importance of Certification in Advancing Careers
Whether you choose to pursue a career as a CRC or CRA, gaining the appropriate certification can significantly boost your career prospects. Specialized certification programs, such as those provided by CCRPS, offer tailored training that can help you stand out in the competitive clinical research industry.
Course Links:
Reference Links:
National Institutes of Health - Clinical Trials - Comprehensive resource for clinical trial information and research.
Association of Clinical Research Professionals (ACRP) - Industry-recognized certifications and educational resources for clinical research professionals.
Society of Clinical Research Associates (SOCRA) - Offers certification and training for clinical research professionals, including CRCs and CRAs.
ICH GCP Guidelines - Internationally accepted guidelines for clinical research best practices, relevant to both CRCs and CRAs.