Clinical Research Associate Requirements
CRA is a challenging and rewarding career that plays an important role in the improvement of medical science and clinical research. CRAs are responsible for ensuring that clinical trials are conducted properly as per the rules and regulations and the protocols set for the same, while protecting the rights of the participants and helping in bringing new treatments and therapies to the market. This blog will aim to provide a comprehensive overview of the types of training, education, and skills that are required to become a CRA, as well as other important factors that determine success in this role.
What Is a Clinical Research Associate?
A Clinical Research Associate (CRA) is a person who supervises clinical trials and implements and follows the procedures and rules. CRAs report to the sponsors, clinical trial sites, and regulatory bodies to guarantee the accuracy and safety of clinical research. They are involved in all stages of the clinical trial process, including the planning and initiation of the trial, conduct of the trial, monitoring of the trial, and reporting of the trial results. CRA is usually represented by pharmaceutical companies, Contract Research Organizations (CRO), or academic institutions.
Educational Requirements for Becoming a Clinical Research Associate
One of the primary requirements for becoming a CRA is to get the required educational qualifications. Most positions demand a minimum of a bachelor’s degree in life science or health related field, though some employers may prefer candidates with advanced degrees or certifications. Here are some common educational pathways to becoming a CRA:
Bachelor’s Degree: A large number of CRAs have a degree in life sciences like biology, chemistry, biochemistry or nursing. A degree in these fields offers the basic knowledge needed to grasp the scientific principles involved in clinical research.
Advanced Degrees: Necessary or not, a master’s or doctoral degree in life sciences or clinical research will not only improve job opportunities. Advanced degrees are evidence of more thorough knowledge and experience in clinical trials, protocols and patient care.
Specialized Certification: To build on the CRA's skills some CRAs may decide to go for certifications in clinical research. These certifications are typically provided by organizations like Association of Clinical Research Professionals (ACRP) or Society of Clinical Research Associates (SOCRA) to validate the advanced knowledge and commitment to the profession in the field.
Clinical Research Coordinator Certification: For the clinical research career, certifications like the Clinical Research Coordinator Certification can improve candidates’ skills and knowledge to make them more marketable in job seeking.
Certification Requirements for Clinical Research Associates
Formal certification is not always required, however it is strongly advised that a CRA become certified. Certification of a CRA implies familiarity with clinical research regulations, protocols, and ethical concerns. It may also help secure a job or get a better position in the career ladder when applying for jobs. Below are some of the most recognized certifications for Clinical Research Associates:
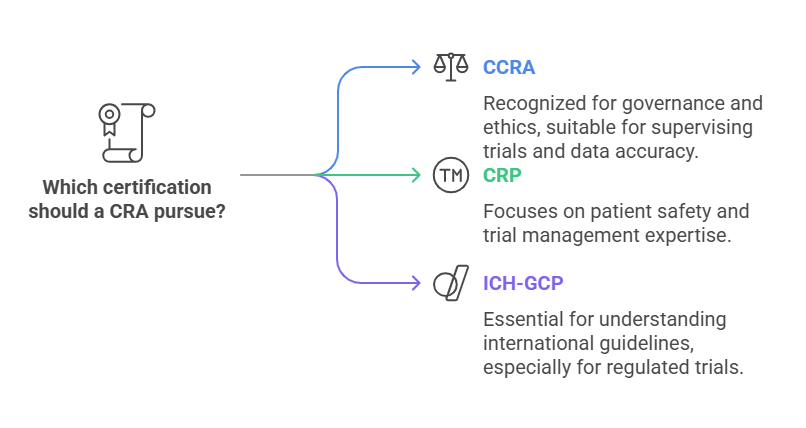
Certified Clinical Research Associate (CCRA): This certification is offered by the ACRP and it stands for Certified Professional in Research Governance & Ethics (CPRGE). This certification assures an external auditor that the CRA is capable of supervising clinical trials, maintaining data accuracy, and adhering to GCP guidelines.
Clinical Research Professional (CRP): This certification is administered by SOCRA and is designed for individuals who wish to have their expertise in clinical research certified, including in such areas as patient safety and trial management.
ICH-GCP Certification: This certification assures that CRAs are conversant with the ICHs (International Council for Harmonisation) guidelines on Good Clinical Practice (GCP) that are crucial in conducting safe and ethical clinical trials. ICH-GCP certification is usually a necessity for employment in clinical trials that are regulated, and particularly for multinational trials, because of their cross-border nature.
Skills and Competencies Required for CRAs
Beside the education and certification, the successful CRAs should possess certain soft and hard skills. These skills help them to deal with the complexity of the clinical trials and make sure that the trial is conducted according to the rules and regulations. Key skills include:
1. Attention to Detail
Clinical trials are characterized by accurate procedures, many regulations and much documentation. CRAs are accountable for making sure that every part of the trial, right up to participant identification to data collection, is correct. It is a precise institution, one that could be derailed by a single wrongful assumption, so attention to detail is crucial.
2. Communication Skills
CRAs are usually the main communication between trial sites, sponsors, and regulatory agencies. They must have a good communication skill set to pass information accurately and in a simple manner. Whether in the form of reports or when replying to questions from trial staff, CRAs have to make sure that everyone involved is up to date and in the loop.
3. Regulatory Knowledge
CRAs must know clinical research regulations like FDA guidelines, ICH-GCP standards, and other international regulations that apply. They can guarantee that all trial activities reflect ethical and legal protection of participants and the research by using this knowledge.
4. Project Management
It is essential to have strong organizational and project management skills to handle a clinical trial. CRAs must be able to control several aspects of the trial, including the budget and resources, meeting deadlines, and the timely reporting of data. These project management skills make sure that the trials are run effectively and in compliance with the regulations.
5. Problem-Solving
Problems are inevitable in clinical trials whether it be in the area of participant recruitment, data collection or protocol deviations. The CRAs have to display critical thinking and find a way to solve these problems while at the same time upholding the credibility of the trial and meeting the regulatory requirements.
Experience Requirements for Clinical Research Associates
Besides education and certification, there is usually a need for some hands-on experience in clinical research or related fields to qualify for a CRA position. Employers typically look for applicants who have worked in positions like Clinical Research Coordinators (CRCs) or assistants, before being ready to work as CRAs. These roles offer familiarity with the operational aspects of clinical trials – patient recruitment, data management, and regulatory compliance – to the incumbent.
Experience can also be gained through internships or entry level positions within pharmaceutical companies, CROs, or even academic research institutions. Many entry level CRAs get on the job training and mentorship, which enables them to gain practical experience while working under the supervision of more experienced CRAs.
Career Advancement and Growth Opportunities
Clinical research is a fast growing field and there is a great need for qualified CRAs. With the continuing complexity and growth of clinical trials CRAs with the right skills and experience have many opportunities for career growth. Here are some potential career pathways for experienced CRAs:
Senior Clinical Research Associate: Senior CRAs, with extra experience and certification, supervise more complicated trials and hold more accountability.
Clinical Trial Manager: CRAs with strong leadership and project management skills may progress to the role of Clinical Trial Managers (CTMs), managing complete trials or many trials at a time.
Director of Clinical Operations: For the very experienced and skilled, clinical operations management roles can provide leadership, overseeing the entire clinical research department or organization.
Embarking on a Career as a Clinical Research Associate
Working as a Clinical Research Associate is a great job with a good growth potential. CRAs are there to make sure that clinical trials are done in a safe and efficient manner so that new treatments and therapies can be brought to the market. Whether you are new to the path or seeking to grow in the profession, it is important to meet the educational, certification, and skill needs for success.
You can set yourself up for a fulfilling career as a CRA by getting the needed education, having the right experience and getting certification. You can be part of the exciting world of clinical research, helping to define the future of medicine and patient outcomes worldwide with the right foundation.
Conclusion
In conclusion, embarking on a career as a Clinical Research Associate (CRA) is a fulfilling path that offers extensive opportunities for growth and advancement in the field of medical science. By acquiring the necessary education, gaining hands-on experience, and seeking certification, such as those offered by recognized organizations like the Clinical Research Professionals Society (CCRPS), aspirants can enhance their qualifications and career prospects. The role of a CRA is crucial in ensuring the efficacy and safety of clinical trials, contributing significantly to the development of new medical treatments. With dedication and the right credentials, CRAs can expect a dynamic and impactful career. Pursuing CCRPS certification can be a pivotal step in setting oneself apart in this competitive field.
Relevant Course Links:
Reference Links:
National Institutes of Health (NIH) - Clinical Research Training and Medical Education
Association of Clinical Research Professionals (ACRP) - Certified Clinical Research Associate Certification
U.S. Food and Drug Administration (FDA) - Good Clinical Practice Guidelines
Society of Clinical Research Associates (SOCRA) - Clinical Research Professional Certification
World Health Organization (WHO) - Clinical Trials and Regulatory Guidelines
Frequently Asked Questions (FAQs)
-
The starting salary for a Clinical Research Associate can vary based on factors like location, employer type, and level of education. Generally, entry-level CRAs can expect to earn between $45,000 and $60,000 annually in the United States. Salaries can increase significantly with experience, additional certifications, and advancement into senior roles.
-
Becoming a Clinical Research Associate typically requires obtaining a bachelor's degree in a life science or related field, which takes about four years. After completing the degree, gaining relevant clinical research experience through roles such as Clinical Research Coordinator or assistant is often necessary, which can take an additional 1 to 2 years. Pursuing certification, which can further enhance employability, requires at least two years of professional experience in a clinical research setting.
-
Certification is not mandatory for all CRA positions, but it is highly recommended. Certified Clinical Research Associates often have better job prospects and potential for career advancement. Certifications such as the Certified Clinical Research Associate (CCRA) from the Association of Clinical Research Professionals (ACRP) or the Clinical Research Professional (CRP) from the Society of Clinical Research Associates (SOCRA) are recognized in the industry.
-
Daily responsibilities of a Clinical Research Associate include monitoring clinical trial sites to ensure compliance with study protocols, FDA regulations, and ethical standards; managing study documentation and patient data; coordinating with clinical trial staff to ensure that trial activities are conducted correctly; and reporting findings to trial sponsors and regulatory bodies. CRAs also address any issues that arise during the trial and ensure that all aspects of the study are accurately documented.
-
Yes, Clinical Research Associates can work remotely, depending on the nature of the clinical trials and the policies of the employer. Remote work may involve tasks such as reviewing data, preparing reports, and conducting meetings with trial staff via virtual platforms. However, CRAs are typically required to travel to clinical trial sites periodically to perform on-site monitoring, which includes inspecting trial conduct and verifying data accuracy directly.