Clinical Research Job Interview Tips: How to Land Your Dream Role?
Whether you are an aspiring Clinical Research Associate (CRA), a seasoned Clinical Trial Manager (CTM), or a Clinical Research Coordinator (CRC) looking to transition into a new organization, nailing your job interview is a vital step toward landing your dream role in the clinical research field. The clinical research industry is highly competitive and regulated, and interviewers are looking for candidates who are not only technically sound but also ethically responsible, detail-oriented, and passionate about improving public health through research.
In this comprehensive guide, we’ll cover everything you need to succeed at your clinical research job interview. From what to wear and how to prepare, to the types of questions you'll be asked and how to stand out from other candidates — this guide has you covered.
Understanding the Clinical Research Job Market
Before heading into interviews, it’s critical to understand the industry landscape. Clinical research is a growing field with a wide variety of roles including:
Clinical Research Associate (CRA)
Clinical Trial Assistant (CTA)
Clinical Data Manager
Regulatory Affairs Specialist
Clinical Project Manager
Clinical Research Coordinator (CRC)
Each of these roles requires a unique skill set and may demand varying levels of education and certifications. While some roles require a background in nursing or life sciences, others prioritize project management and communication skills.
Trends Shaping the Industry:
Decentralized Clinical Trials (DCTs)
AI in Clinical Research
Regulatory changes and global harmonization
Increased demand for certified professionals
Research the Role and Company
Thorough research is one of the most overlooked but essential parts of interview prep.
What to Research:
The organization’s mission, values, and research focus areas
Recent clinical trials or publications
Organizational structure and teams
Regulatory history or compliance accolades
Tip:
Align your personal values and long-term career goals with the company’s mission. This will help you authentically connect during the interview.
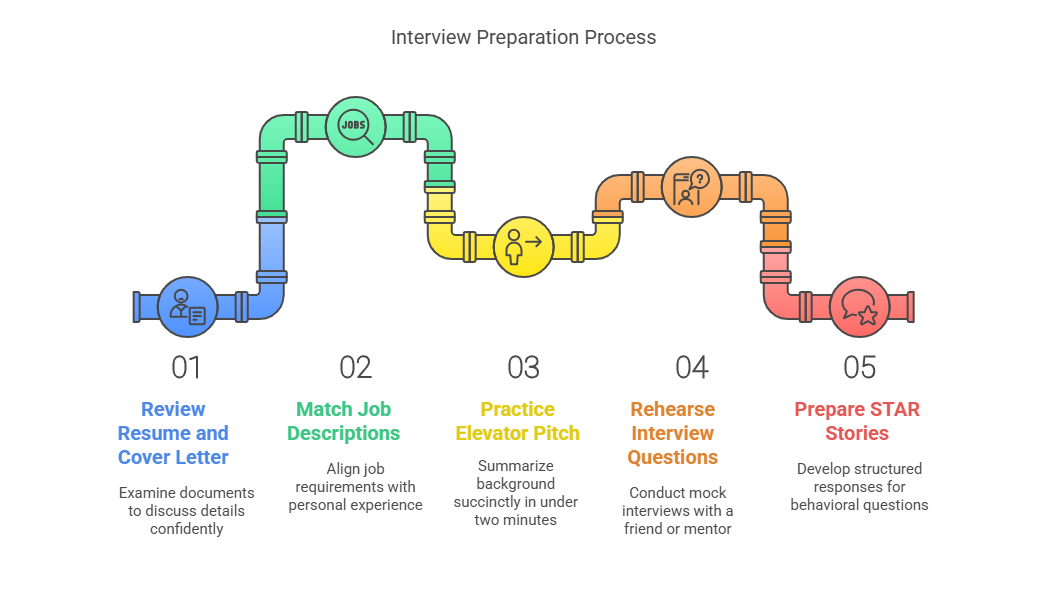
3. Preparing for the Interview
Preparation goes beyond memorizing your resume.
Key Steps:
Review your resume and cover letter — be ready to discuss every detail.
Revisit job descriptions and match them with your experience.
Practice your elevator pitch — summarize your background in under 2 minutes.
Rehearse common interview questions with a friend or mentor.
Prepare STAR (Situation, Task, Action, Result) stories for behavioral questions.
Must-Haves:
Copies of your resume and certifications
List of references
Notebook and pen
Most Common Clinical Research Interview Questions
General Questions:
Tell me about yourself.
Why are you interested in this role?
What do you know about our company?
Role-Specific Questions:
How do you manage source documents?
Explain the difference between Phase II and Phase III trials.
Describe your experience with regulatory submissions.
How do you manage protocol deviations?
Scenario-Based Questions:
What would you do if a PI (Principal Investigator) is not following GCP?
How do you handle discrepancies in data collection?
How to Answer Behavioral Interview Questions
The STAR method is your best friend when answering behavioral questions. It helps you provide a concise and structured response.
Example Question:
"Tell me about a time you had to deal with a difficult team member."
S: One of my previous CTAs frequently missed deadlines.
T: I had to ensure timely submission of documents to meet the trial’s timeline.
A: I scheduled a one-on-one to understand the root of the issue and reallocated tasks based on individual strengths.
R: Document turnaround improved by 30%, and we met our submission deadline.
Demonstrating Soft Skills and Technical Knowledge
Recruiters aren’t just looking for textbook knowledge — they want well-rounded professionals.
Key Soft Skills:
Communication
Time management
Problem-solving
Adaptability
Technical Competencies:
Understanding of ICH-GCP
Familiarity with EDC (Electronic Data Capture) systems like Medidata or Oracle
Experience with CRFs (Case Report Forms)
Adherence to protocol and SOPs
Pro Tip:
Mention certifications or ongoing training courses like GCP certification, Clinical Research Associate Certification (CRAC), etc.
What to Wear and Bring to the Interview
Attire:
Business formal is typically safe — suit, blouse, dress shoes.
Avoid bright colors and overpowering accessories.
What to Bring:
Multiple copies of your resume
A folder with certificates or credentials
A list of thoughtful questions to ask the interviewer
Virtual Interview Tips
In a post-pandemic world, virtual interviews have become the norm.
Tips:
Test your tech: camera, microphone, internet.
Choose a quiet, neutral background.
Dress professionally — even if you’re only seen from the waist up.
Maintain eye contact by looking at the camera, not the screen.
Following Up After the Interview
A thank-you email is not just polite — it’s strategic.
Include:
Appreciation for the interviewer’s time
Key takeaways from the conversation
Reiteration of your interest in the role
Links to your LinkedIn or online portfolio (if relevant)
Send the follow-up within 24 hours of your interview.
For more detailed guidance on getting ready for your interview, check out this guide on clinical research interview preparation.
Final Words of Advice
Practice, but don’t sound scripted.
Be authentic — interviewers can sense pretense.
Prepare examples that showcase both successes and challenges.
Don’t be afraid to ask questions — it shows curiosity and engagement.
If you’re applying for a CRA role, reviewing these interview questions for clinical research monitors can give you a competitive edge.
Conclusion
Landing a clinical research role isn’t just about having the right degree — it’s about demonstrating your readiness, communication skills, and ethical commitment to research. By implementing these strategies and preparing thoroughly, you’ll walk into your interview with confidence.
To gain a competitive edge, consider enrolling in a training or certification program through CCRPS — a trusted provider of clinical research education designed to help you advance your career.
-
A background in life sciences, nursing, or public health is often required. Certifications like GCP or CRA are highly valued.
-
Tailor your responses to the job description, show enthusiasm, and demonstrate knowledge of current trends in clinical trials.
-
Expect questions on monitoring visits, protocol adherence, and data quality assurance.
-
Only if the interviewer brings it up. Otherwise, wait until later stages.
-
Very. Certifications signal commitment and competence, especially for entry-level roles.
-
Typically 30-60 minutes, though panel interviews may last longer.
-
Yes, especially if you highlight transferable skills and obtain relevant training.
-
CCRPS offers certified courses, resume support, and career guidance tailored for aspiring clinical research professionals.