How to Become a Clinical Project Manager?
It is a rewarding career path to become a clinical project manager (CPM) as one gets to manage and lead clinical trials to success and in accordance with the set rules and regulations. Clinical project managers are important in the healthcare industry; they help in the implementation of new medical innovations. If you are planning to pursue this career, following is a comprehensive guide on how to go about it, the educational and work experience requirements, and the characteristics that will enable you to excel in this position.
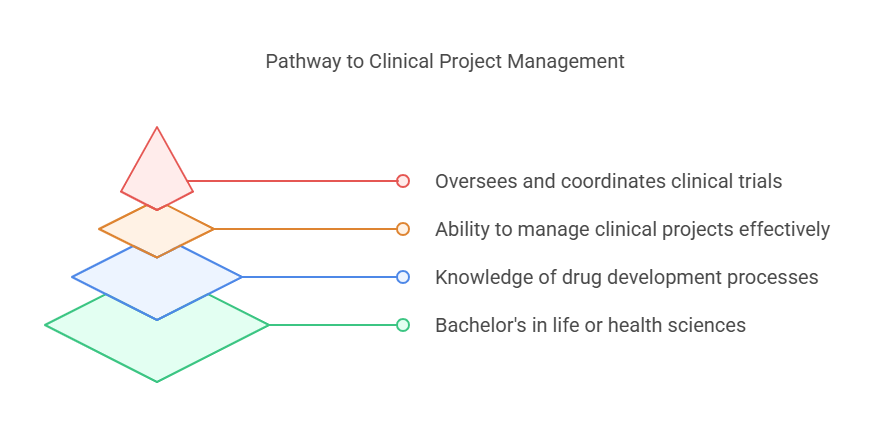
Step 1: Relevant Bachelor's Degree
The first way to become a clinical project manager is to get a bachelor’s degree in any of the following fields: Life sciences, Health sciences, Biology, Chemistry, or Pharmacology. There is no such a degree called Clinical Project Management but the following are some of the common degrees; life sciences, health sciences, biology, chemistry or pharmacology. With these fields of study, one would be in a position to understand clinical trials and the drug development process.
Popular Undergraduate Degrees for Clinical Project Managers:
Biology: Offers a deep understanding of human biology and laboratory practices.
Chemistry: Focuses on drug formulation and chemical processes.
Nursing: Provides insight into patient care and clinical settings.
Public Health: Covers broader health issues and epidemiology, which are relevant to clinical trials.
Moreover, some students opt for business administration or project management degrees to advance their understanding of large-scale project management.
Step 2: Experience in Clinical Research
A very important step in the process of becoming a clinical project manager is to have experience in the clinical research field. Many begin their careers in entry level positions like clinical research coordinators (CRC), clinical trial assistants (CTAs), or clinical research associates (CRAs). These positions enable you to gain knowledge of clinical trials, from patient recruitment to data collection to regulatory compliance.
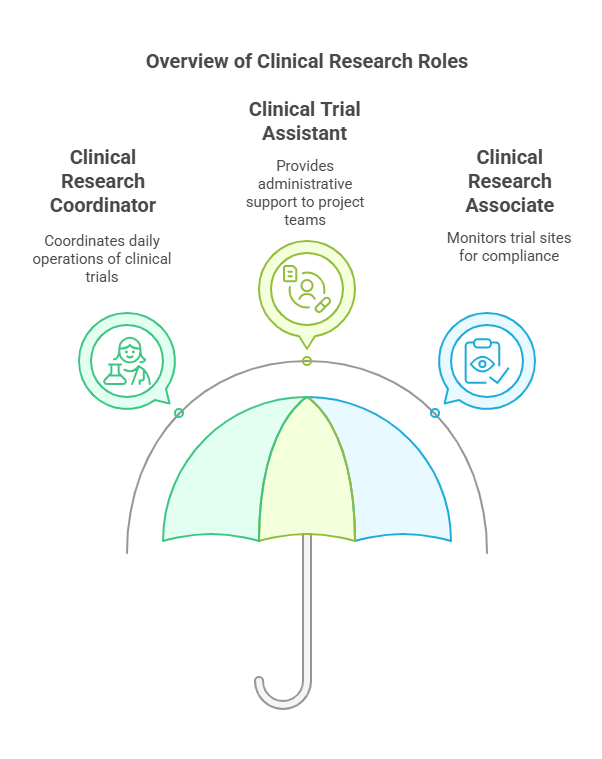
Common Entry-Level Roles:
Clinical Research Coordinator (CRC): Coordinates the daily operations of clinical trials.
Clinical Trial Assistant (CTA): Provides administrative support to project teams.
Clinical Research Associate (CRA): Monitors trial sites to ensure compliance with protocols and regulations.
You get to learn the regulatory framework and the operational challenges of a clinical project manager, which you will be eventually overseeing in these roles. Valuable insights into patient management, protocol adherence, and communication with study stakeholders are also gained through experience in these positions.
For those looking to jumpstart their career, they should consider enrolling in a specialized course such as the Clinical Research Coordinator training or the Clinical Trials Assistant Training course, in order to obtain the skills and certification needed to break into the field.
Step 3: Certifications and Advanced Training
Although experience in clinical research is significant, certifications are becoming a desired qualification for employers when seeking to recruit clinical project managers. Obtaining certifications in clinical research management is proof of your continuing professional development and your expertise.
Some of the most recognized certifications include:
Project Management Professional (PMP): Developed by the Project Management Institute (PMI), this certification is relevant to basic project management across all industries with a specific focus on clinical research.
Certified Clinical Research Professional (CCRP): This certification is specifically focused on clinical research professionals and is provided by the Society of Clinical Research Associates (SOCRA).
Certified Clinical Project Manager (CCPM): A specialized certification targeted at the specific obligations and complexities of clinical trial management is this one.
To find out more about the regulatory and operational aspects of clinical trials, you should consider entering advanced courses such as the Advanced Clinical Research Project Manager Certification or CRA training to gain the skills needed to excel in this role.
Step 4: Develop Essential Skills for Clinical Project Management
Successful clinical project managers possess a unique combination of scientific knowledge, leadership abilities, and organizational skills. Here are some of the key skills required:
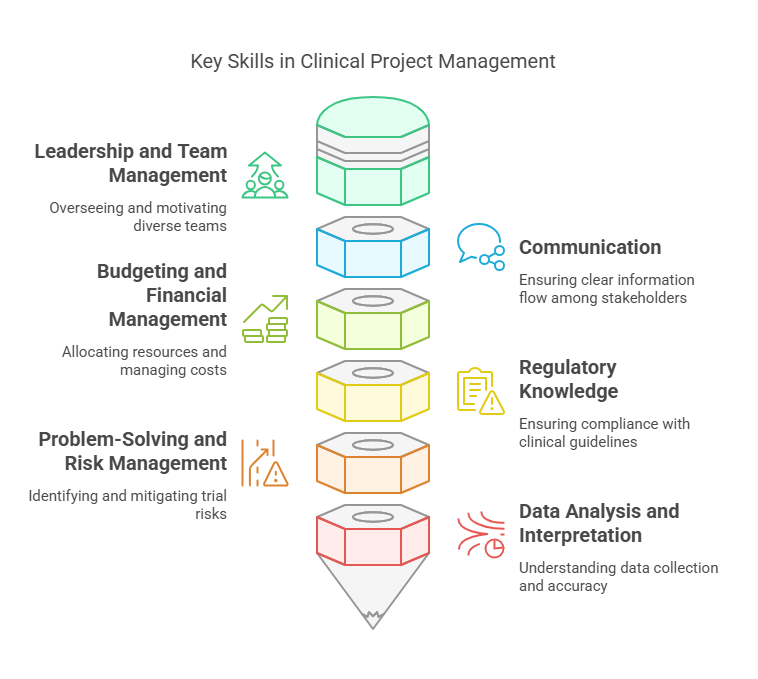
Leadership and Team Management:
CPMs oversee multidisciplinary teams, including scientists, clinicians, and regulatory experts. Strong leadership skills are needed to motivate, coordinate, and manage these diverse teams.
Communication:
CPMs must be excellent communicators, as they often serve as a liaison between research teams, sponsors, and regulatory authorities. Effective communication ensures that all stakeholders are informed and aligned on the trial’s progress.
Budgeting and Financial Management:
Managing the financial aspects of clinical trials, including budgeting and resource allocation, is a critical responsibility. CPMs must ensure that the project stays within budget without compromising the quality of the research.
Regulatory Knowledge:
Clinical trials are governed by strict regulations, including the International Council for Harmonisation (ICH) guidelines and Good Clinical Practice (GCP) standards. CPMs must stay up-to-date with these regulations to ensure compliance throughout the trial.
Problem-Solving and Risk Management:
Clinical trials often encounter unforeseen challenges, such as patient recruitment delays or data inconsistencies. CPMs must be skilled in identifying potential risks early on and developing strategies to mitigate them.
Data Analysis and Interpretation:
While not directly responsible for analyzing trial data, CPMs must have a thorough understanding of how clinical data is collected and interpreted to ensure that study results are accurate and reliable.
Step 5: Gain Experience in Project Management
In addition to clinical research experience, it is essential to gain hands-on experience in managing projects. This can be done by taking on leadership roles within clinical trials or managing smaller projects under the guidance of an experienced clinical project manager.
Many clinical project managers start in roles such as associate project managers, where they learn the skills needed to oversee timelines, budgets, and team coordination. This experience prepares them for the more complex responsibilities of leading entire clinical trials.
Step 6: Pursue a Master’s Degree (Optional but Beneficial)
While a bachelor’s degree is sufficient for entry-level roles, a master’s degree in clinical research, project management, or business administration can significantly enhance your prospects of becoming a clinical project manager. Graduate programs provide advanced knowledge in research methodologies, project management strategies, and regulatory affairs, equipping you for higher-level positions.
Some institutions offer specialized master's degrees in clinical research management or healthcare project management. These programs are designed to provide the skills needed to oversee clinical trials effectively and prepare for leadership roles in the field.
Step 7: Network and Stay Current with Industry Trends
The healthcare and clinical research industries are constantly evolving, with new regulations, technologies, and methodologies emerging regularly. Staying informed about these changes is crucial for clinical project managers. Attending industry conferences, webinars, and continuing education courses can help you stay updated on the latest trends.
Networking is equally important. Building relationships with other professionals in the field can provide opportunities for career advancement, mentorship, and collaborative projects. Consider joining professional organizations such as the Association of Clinical Research Professionals (ACRP) or the Society of Clinical Research Associates (SOCRA) to stay connected with the latest developments in the industry.
Career Outlook and Opportunities for Clinical Project Managers
The demand for clinical project managers is growing due to the increasing number of clinical trials worldwide, particularly in areas like oncology, cardiology, and neurology. According to the U.S. Bureau of Labor Statistics, employment in clinical research is expected to grow faster than the average for all occupations over the next decade.
Clinical project managers can work for:
Contract Research Organizations (CROs): These organizations are often contracted by pharmaceutical companies to manage clinical trials.
Pharmaceutical and Biotech Companies: Clinical project managers may work directly for drug development companies to manage in-house trials.
Academic and Research Institutions: Universities and research centers also conduct clinical trials and require CPMs to manage them.
Conclusion
Becoming a clinical project manager requires education, experience, and specialized skills. It’s a rewarding role that impacts medical research and patient care. CCRPS offers advanced certifications to help you excel in clinical project management and healthcare innovation.
Reference Links:
U.S. National Library of Medicine – Clinical Research Careers
Harvard T.H. Chan School of Public Health – Master's in Clinical Research
Project Management Institute (PMI) – Project Management Professional (PMP) Certification
Society of Clinical Research Associates (SOCRA) – Certification Programs