What Is A Clinical Research Associate
A Clinical Research Associate (CRA) plays an important role in medical research by making sure that clinical tests are done properly. These trials test new medicines, treatments, and medical devices to check if they are safe and effective before they reach the public. A CRA helps make sure that every step follows strict rules and guidelines. They work with doctors, scientists, and research teams to keep the study on track and make sure all the necessary instructions are followed correctly. Their job is important because it helps speed up the process of bringing new and improved treatments to people who need them.
A CRA is responsible for visiting hospitals, clinics, and research centers to watch over the progress of a trial. They check medical records, review collected data, and make sure that patients taking part in the study are safe and well-informed. Since clinical trials involve human participants, a CRA ensures that they understand the study and give proper consent before taking part. They also act as a link between research teams and sponsors, such as pharmaceutical companies, to keep them updated on the study’s progress and any important findings.
Their work is important because any mistake or delay in a trial can slow down medical advancements. CRAs help prevent errors, making sure that data is accurate and that trials meet all safety and ethical standards. Without their careful monitoring, new treatments could take longer to be approved, affecting patients who depend on new medicines and therapies. By ensuring that trials run smoothly, CRAs play a key role in improving healthcare and making medical advancements possible.
What Does A Clinical Research Associate Do?
A Clinical Research Associate (CRA) is responsible for making sure that clinical trials are carried out correctly from start to finish. They visit hospitals, clinics, and research centers to observe the progress of a study, check patient records, and make sure that all steps follow the approved guidelines. Their job includes making sure that patients are safe, informed, and treated according to the study’s plan. A CRA also trains research staff on how to follow trial procedures, helping them understand the correct way to collect data and report results.
Another important task of a CRA is ensuring that clinical trials meet all legal and honorable rules. They carefully review data to check for mistakes and make sure that all reports are correct before they are presented. CRAs also communicate with sponsors and regulatory bodies to provide updates and address any issues. Their role is critical in keeping clinical trials organized, ensuring that results are trustworthy, and helping bring safe and effective treatments to the public.
Required Skills And Qualifications
Becoming a Clinical Research Associate (CRA) is a rewarding career for those interested in the medical and research fields. This role requires a mix of education, practical skills, and attention to detail to ensure clinical trials are carried out effectively and ethically. CRAs are essential in overseeing the progress of clinical studies and ensuring compliance with all relevant regulations. If you want to pursue this career, understanding the educational background, key skills, and certifications required is necessary. Below is a detailed breakdown of what you need to succeed in this role.
| Category | Details |
|---|---|
| Education | A degree in life sciences (biology, chemistry), pharmacy, nursing, or related fields is essential. Some employers may prefer candidates with a master's degree, particularly for more advanced research roles. Higher qualifications can make candidates more competitive in the job market. |
| Technical Knowledge | CRAs must understand clinical trial protocols, Good Clinical Practice (GCP) guidelines, and ethical research standards. This knowledge ensures that studies are compliant with both local and international regulations. Understanding the drug development process and patient safety is critical in this role. |
| Attention To Detail | CRAs must pay close attention to every part of a clinical trial, from checking patient records to ensuring that research data is accurate and complete. Mistakes in the data or patient information could lead to delays or weak results, so being careful is a must. |
| Communication Skills | CRAs interact with many people, including researchers, doctors, patients, and sponsors. Clear written and verbal communication is essential to keep everyone on the same page, explaining complex research details, resolving issues, and providing updates about trial progress. |
| Analytical Thinking | Strong problem-solving skills are needed to evaluate trial data and make informed decisions. CRAs must quickly identify discrepancies, evaluate the validity of data, and suggest improvements to trial procedures to keep studies on track. |
| Certifications | While not always required, certifications like the Certified Clinical Research Professional (CCRP) from CCRPS can make candidates stand out. This certification demonstrates that a CRA has the specialized knowledge and training required to manage clinical trials effectively. Being CCRP-certified shows a commitment to high industry standards and enhances a candidate’s credibility, making them more competitive in the job market. |
| Work Experience | While some entry-level positions exist, most employers prefer candidates with some experience in clinical research or related fields, such as nursing or pharmacy. Experience helps CRAs become familiar with the practical aspects of running a clinical trial and working in clinical settings. |
Work Environment And Career Opportunities
The work environment for a Clinical Research Associate (CRA) can vary depending on the industry and the type of study they are involved in. CRAs are typically employed by pharmaceutical companies, biotechnology firms, contract research organizations (CROs), or hospitals. Pharmaceutical and biotech companies often hire CRAs to oversee the clinical trials of new drugs and medical devices. CROs play a key role in managing clinical trials on behalf of pharmaceutical companies, providing a wide range of opportunities for CRAs to gain experience across various studies. Additionally, hospitals or research centers may hire CRAs to ensure that trials run smoothly at the local level, ensuring patient safety and regulatory compliance.
As for career progression, there are several paths a CRA can take as they gain more experience and expertise. Starting as a junior or entry-level CRA, you will typically be responsible for monitoring trials, ensuring regulatory compliance, and reporting on progress. Over time, as you gain experience, you can advance to a Senior CRA position, where you will take on more complex trials and have greater responsibility in terms of oversight and decision-making. From there, many CRAs move into roles like Clinical Project Manager (CPM), where they manage entire clinical trial projects, oversee budgets, and lead teams of CRAs, researchers, and other professionals.
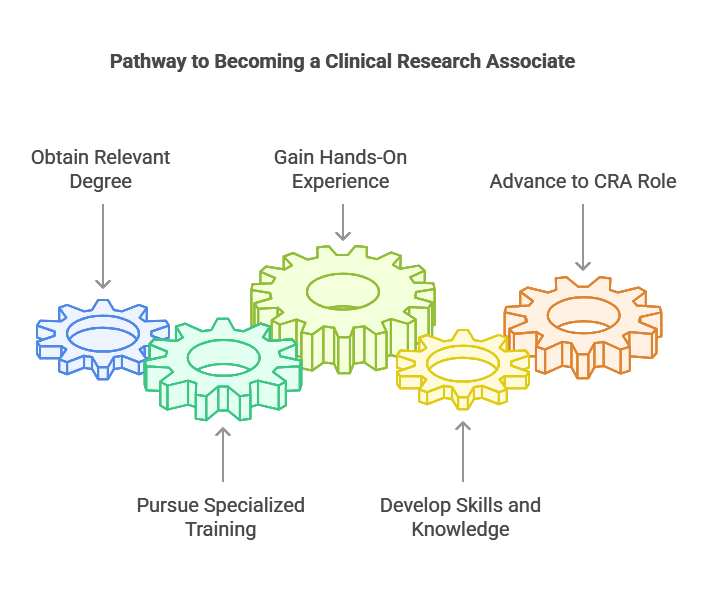
How To Become A Clinical Research Associate
Becoming a Clinical Research Associate (CRA) typically starts with the right educational background. To enter this field, you will need a degree in a relevant field such as life sciences, pharmacy, nursing, or medical research. Some universities also offer specialized courses or certifications in clinical research, which can provide additional knowledge and make you a more competitive candidate. After completing your degree, you may want to pursue further training in clinical trial management or Good Clinical Practice (GCP) guidelines, which are essential for ensuring the trials are carried out properly and ethically.
Once you have the educational foundation, gaining hands-on experience is essential. Many entry-level CRAs start with positions like Clinical Research Coordinator (CRC) or Research Assistant, where they support clinical trials and learn about trial protocols, data collection, and patient management. These roles allow you to develop the necessary skills while gaining a deeper understanding of clinical research. By working closely with experienced CRAs and learning the ropes, you will be better prepared for more advanced roles, such as monitoring trials independently or moving into senior CRA positions. Experience is key, as it helps you build the necessary skills and credibility in the field.
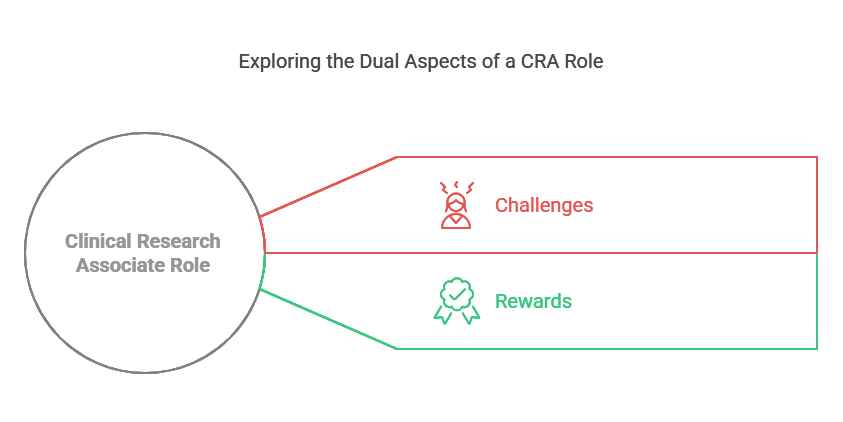
Challenges And Rewards Of The Role
The role of a Clinical Research Associate (CRA) offers both significant challenges and great rewards. CRAs play a vital role in the medical research process, but their work can be demanding. Some of the challenges include tight deadlines and frequent travel to trial sites. On the flip side, the role is also rewarding, offering high job demand, career growth, and competitive salaries. If you want to dive deeper into the specifics, keep reading to learn more about the challenges and rewards that come with being a CRA.
Challenges Of The Clinical Research Associate Role:
Tight Deadlines: One of the biggest challenges CRAs face is managing tight deadlines. Clinical trials are often time-sensitive, and there are many moving parts to keep track of. CRAs must ensure that patient data is accurately recorded, trial progress is reported in real-time, and compliance with regulations is maintained, all within strict timelines. This pressure can be overwhelming at times, especially when handling multiple trials altogether. Additionally, unexpected issues, such as delays or protocol changes, can make the process even more challenging. Meeting deadlines without compromising on quality or safety is a skill CRAs must develop to thrive in the role.
Travel Requirements: Another challenge for CRAs is the travel requirements. The role often requires visits to different trial sites, which can be both time-consuming and tiring. CRAs must travel to ensure that all trial protocols are being followed and to monitor patient safety, which may involve long hours and days away from home. The travel burden is particularly heavy for those managing multi-site trials or working in remote locations. While some people enjoy the variety and excitement of frequent travel, others may find the constant movement difficult to manage, especially when balancing work with personal life.
Rewards Of The Clinical Research Associate Role:
High Demand: A significant reward for being a CRA is the high demand for qualified professionals in the industry. As the need for clinical trials increases, CRAs are in greater demand across sectors like pharmaceuticals, biotech, and hospitals. This demand provides job security, as companies and research organizations require skilled CRAs to oversee the smooth running of their trials. As the healthcare industry continues to expand, the role of a CRA becomes even more essential, ensuring that there will be opportunities for career advancement and job stability.
Career Growth And Competitive Salary: Another major reward of being a CRA is the potential for career growth. With experience, CRAs can progress to senior roles like Senior CRA or Clinical Project Manager, where they will take on more responsibility and earn a higher salary. The role also offers competitive pay, with compensation increasing with experience and specialization. CRAs enjoy the benefit of working in a field with strong career progression, where they can advance to leadership positions or take on more complex, high-profile clinical trials. Additionally, the sense of contributing to meaningful medical research is a major professional reward.
Conclusion
A Clinical Research Associate (CRA) plays a vital role in overseeing clinical trials that test new treatments, medicines, and medical devices. They ensure that each trial is conducted according to strict regulations, protecting patient safety and ensuring accurate data collection. CRAs work closely with doctors, researchers, and sponsors to monitor trial progress and maintain high standards of compliance throughout the process. While the job can be demanding, with tight deadlines and travel requirements, the role also offers numerous rewards. The growing demand for CRAs ensures job security, and with experience, professionals can advance to senior positions with higher salaries. This career also provides the satisfaction of contributing to groundbreaking medical research that can improve lives. With the right education and training, becoming a CRA offers exciting opportunities for growth in the healthcare and research sectors.
Elevate your career in clinical research with CCRPS, a leader in cutting-edge education and professional certification. Founded in 2016, we provide advanced programs designed to equip you with the expertise and real-world skills needed to thrive in clinical research. Our accredited courses, including Clinical Research Associate, Pharmacovigilance, and Clinical Project Manager, are trusted by over 1,576 hiring organizations globally. Whether you're just starting or advancing your career, CCRPS offers the training and mentorship to help you succeed. Join us and become part of a global network of professionals transforming the future of clinical research. For more details, contact us at support@ccrps.org or call +1 (239) 329-9837. Visit our website for more information and start your journey today!