Why Clinical Trials Day Matters for Healthcare Innovation
Clinical Trials Day, observed annually on May 20th, is a global celebration of clinical research and its role in advancing healthcare innovation. It commemorates the day in 1747 when James Lind conducted one of the first controlled clinical trials aboard a British naval ship, leading to the discovery that citrus fruits could prevent scurvy. This landmark study laid the foundation for the rigorous clinical trials we rely on today to evaluate new treatments, therapies, and interventions.
Clinical Trials Day not only honors past achievements but also shines a spotlight on the essential work of researchers, healthcare professionals, and the volunteers who make these studies possible. As we look to the future, the importance of clinical trials in driving healthcare innovation cannot be overstated. This blog will explore why Clinical Trials Day is significant for the medical community, the role clinical trials play in advancing healthcare, and how this annual celebration helps raise awareness about the importance of research participation.
What is Clinical Trials Day?
Clinical Trials Day is an opportunity to recognize the critical contributions that clinical trials make to medical advancements. The day is marked by events, educational programs, and outreach efforts that aim to increase public understanding of clinical trials and encourage participation in ongoing research. From the development of new drugs to innovations in medical devices and treatments for chronic diseases, clinical trials are the cornerstone of evidence-based healthcare.
Organizations around the world, including hospitals, universities, and research institutions, use this day to celebrate the progress made in medical research and to promote the importance of ongoing clinical trials. It is a time to reflect on the successes of the past, acknowledge the challenges that remain, and inspire a new generation of researchers and participants to continue the work of advancing healthcare.
The Importance of Clinical Trials in Healthcare Innovation
Clinical trials are essential to the development of new medical treatments and therapies. They provide the scientific data needed to determine whether a new drug or treatment is safe and effective for human use. Without clinical trials, healthcare innovation would stagnate, and patients would not have access to the latest advancements in medical care.
Here are some key reasons why clinical trials are critical for healthcare innovation:
1. Testing New Treatments and Therapies
Clinical trials are the primary way in which researchers evaluate the safety and effectiveness of new treatments. Whether it’s a new drug, a surgical procedure, or a medical device, clinical trials provide the data needed to bring these innovations to market. Before a treatment can be approved by regulatory bodies like the FDA or EMA, it must undergo rigorous testing in clinical trials to ensure that it works as intended and doesn’t pose undue risks to patients.
Phase I Trials: These early trials test the safety of a new treatment in a small group of participants. They help determine the appropriate dosage and identify any potential side effects.
Phase II Trials: In this phase, the treatment is tested on a larger group to assess its effectiveness and further evaluate its safety.
Phase III Trials: These large-scale studies compare the new treatment to standard treatments, providing a comprehensive understanding of its benefits and risks.
Through these phases, clinical trials ensure that new treatments are both safe and effective before they are made widely available to the public. Without these studies, healthcare would rely on anecdotal evidence or unproven treatments, which could put patients at risk.
2. Advancing Personalized Medicine
One of the most exciting areas of healthcare innovation is personalized medicine, which tailors treatments to individual patients based on their genetic makeup, lifestyle, and other factors. Clinical trials are integral to the development of personalized medicine, as they help researchers understand how different patients respond to treatments. By analyzing trial data, researchers can identify which treatments are most effective for specific patient populations, leading to more targeted and effective healthcare interventions.
For example, in cancer research, clinical trials have been instrumental in developing targeted therapies that attack cancer cells based on specific genetic mutations. These treatments have transformed the way we approach cancer care, offering more effective and less harmful alternatives to traditional chemotherapy.
Personalized medicine holds the promise of reducing trial and error in treatment selection and improving patient outcomes. As this field grows, the need for well-designed clinical trials to validate new personalized treatments will continue to increase.
3. Ensuring Safety and Efficacy
Patient safety is the top priority in any clinical trial. Before a new treatment can be approved for widespread use, it must pass through multiple phases of testing to ensure that it is both safe and effective. Clinical trials adhere to strict protocols and regulatory standards, including Good Clinical Practice (GCP) guidelines, to protect participants and maintain the integrity of the data collected.
The role of clinical trials in ensuring safety cannot be overstated. Many treatments that appear promising in preclinical studies fail in clinical trials because they are found to be unsafe or ineffective. By thoroughly testing treatments before they are approved, clinical trials prevent potentially harmful interventions from reaching the market and ensure that patients receive the best possible care.
For those interested in learning more about GCP and the ethical considerations involved in clinical trials, this ICH GCP Course provides comprehensive training on regulatory compliance in clinical research.
4. Driving Innovation Through Collaboration
Clinical trials are often a collaborative effort between pharmaceutical companies, research institutions, healthcare providers, and regulatory agencies. This collaboration fosters innovation by bringing together diverse expertise and resources to address complex medical challenges. By working together, these stakeholders can accelerate the development of new treatments and ensure that they are accessible to patients as quickly as possible.
In recent years, the collaboration between researchers and pharmaceutical companies has been crucial in the rapid development of vaccines and treatments for COVID-19. Clinical trials were conducted at an unprecedented speed, thanks to global collaboration and innovative trial designs. This collaborative approach has set a new standard for how we can tackle urgent healthcare needs in the future.
Raising Awareness on Clinical Trials Day
One of the primary goals of Clinical Trials Day is to raise awareness about the importance of clinical trials and encourage more people to participate in research studies. Despite the critical role that clinical trials play in healthcare innovation, many trials struggle to recruit enough participants. This can delay the development of new treatments and prevent researchers from gathering the data needed to move forward with approval processes.
Why Participation Matters
Clinical trials rely on volunteers to test new treatments and gather data on their safety and effectiveness. Without participants, trials cannot proceed, and promising treatments may never reach the market. Participation in clinical trials not only helps advance medical research but also provides participants with access to cutting-edge treatments that may not be available through standard care.
Participants in clinical trials often receive medical care and monitoring as part of the study, and in some cases, they may benefit from receiving a new treatment that proves more effective than existing options. However, it’s important to note that participation in clinical trials is voluntary, and informed consent is a critical part of the process to ensure that participants understand the potential risks and benefits.
For those considering participating in clinical research, understanding the rights and responsibilities of research participants is essential. Resources like this Clinical Trials Assistant Training provide valuable insights into the clinical trial process and help potential participants make informed decisions.
Overcoming Misinformation
Despite the benefits of clinical trials, many people are hesitant to participate due to misconceptions or fears about the research process. Clinical Trials Day serves as an opportunity to dispel these myths and provide accurate information about what clinical trials involve. By educating the public about the rigorous safety protocols in place and the potential benefits of participation, researchers can help build trust and increase participation rates.
Conclusion: Celebrating Innovation and Participation
Clinical Trials Day is more than just a celebration of the past; it is a call to action for the future. As healthcare continues to evolve, clinical trials will remain a cornerstone of medical innovation, providing the data needed to develop safe, effective, and personalized treatments. By raising awareness, promoting participation, and honoring the contributions of researchers and volunteers, Clinical Trials Day plays a vital role in ensuring that medical research continues to advance.
Whether you’re a researcher, a healthcare professional, or a potential participant, Clinical Trials Day is a reminder of the critical importance of clinical research in improving patient outcomes and advancing global healthcare. For those looking to get involved in the clinical research process, consider exploring the opportunities available through training and certification programs like the Advanced Clinical Research Project Manager Certification, which can help you make a meaningful impact in the field of clinical research.
Reference Links:
National Institutes of Health (NIH) - Clinical Trials: What You Need to Know
World Health Organization (WHO) - Clinical Trials
U.S. Food & Drug Administration (FDA) - Clinical Trials Guidance Documents
ClinicalTrials.gov - Clinical Trials Information
Association of Clinical Research Professionals (ACRP) - Clinical Research Training
Relevant Course Links:
Top Benefits of Earning a CRP Certification
In today’s competitive professional landscape, certification programs offer a way to enhance skills, build credibility, and gain recognition in various industries. One certification that has gained considerable traction is the CRP (Certified Research Professional) certification. This certification is particularly valuable for individuals involved in clinical research, as it equips them with the knowledge and skills necessary to excel in the fast-paced, regulated environment of clinical trials and research projects.
This blog will delve into the top benefits of earning a CRP certification, outlining how this credential can help professionals advance their careers, improve their earning potential, and enhance their industry knowledge. If you’re considering pursuing a CRP certification or are simply curious about its advantages, read on to discover how it can provide tangible value to your professional journey.
What is CRP Certification?
CRP certification, or Certified Research Professional certification, is a credential that validates the expertise and competency of professionals in the field of clinical research. It is widely recognized by regulatory bodies, healthcare organizations, and academic institutions as a mark of excellence in clinical research management.
The certification is awarded to individuals who demonstrate a deep understanding of clinical trial management, regulatory compliance, Good Clinical Practice (GCP) guidelines, and ethical considerations in human research. By obtaining CRP certification, professionals signal their commitment to maintaining high standards in clinical research, ensuring that trials are conducted safely, ethically, and efficiently.
1. Career Advancement Opportunities
One of the primary reasons professionals pursue CRP certification is the opportunity it provides for career advancement. The clinical research field is highly competitive, and having a CRP certification on your resume can set you apart from other candidates when applying for higher-level positions. Employers often seek out certified professionals because they have demonstrated the knowledge and skills necessary to manage clinical trials effectively.
Key Benefits for Career Growth:
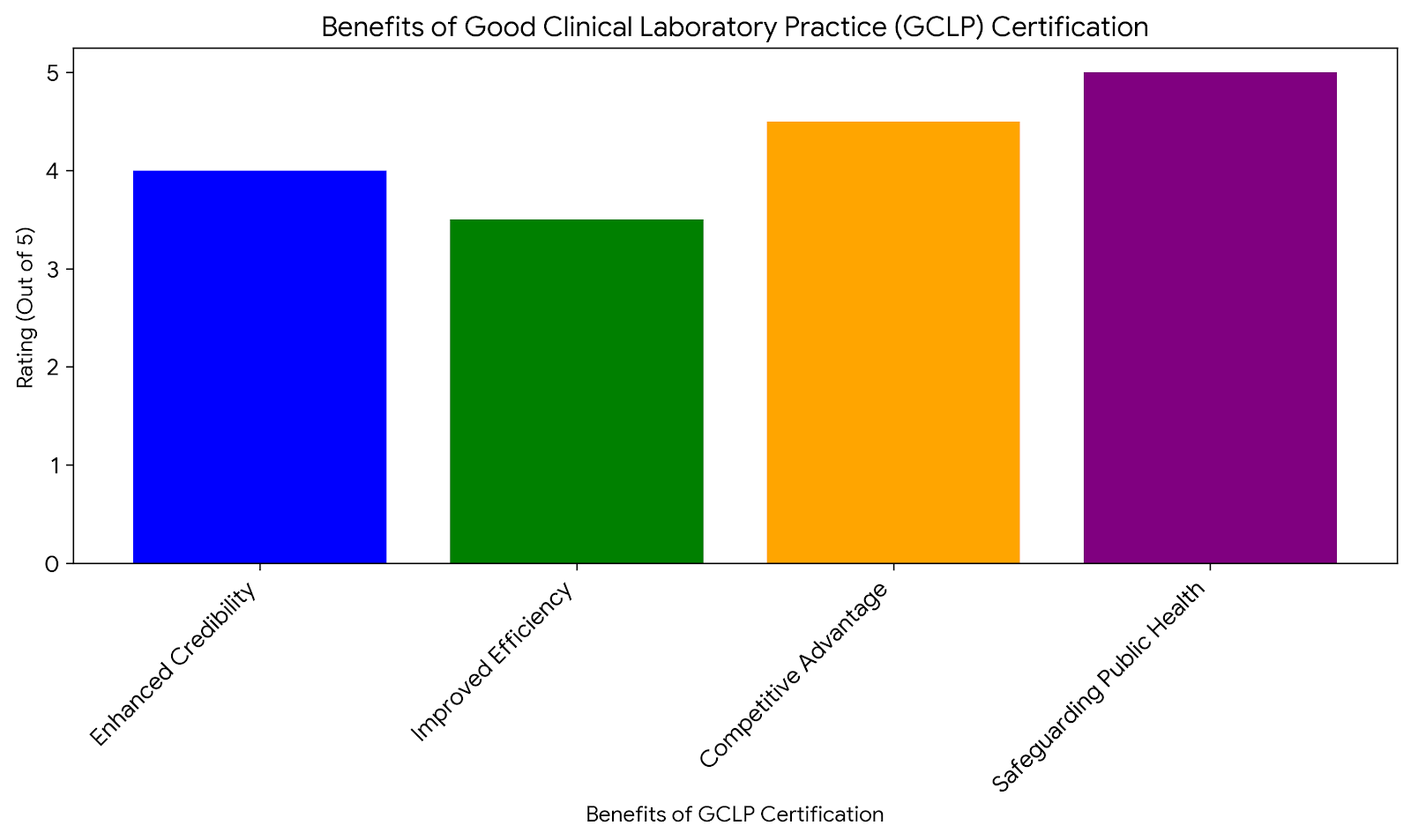
Enhanced Credibility: CRP certification signals to employers and colleagues that you are well-versed in the essential aspects of clinical research, from regulatory compliance to trial management.
Better Job Prospects: With a CRP certification, you are more likely to qualify for positions such as clinical trial manager, clinical research associate, or regulatory affairs specialist, where advanced knowledge of clinical research practices is required.
Increased Job Security: Certification provides an edge in the job market, making you more valuable to employers who are seeking well-qualified professionals to manage their research studies.
For professionals aiming to advance to leadership positions within clinical research, obtaining a CRP certification is often seen as a necessary step. Many employers view this credential as a marker of competence and trustworthiness, which can lead to promotions and higher salaries.
2. Increased Earning Potential
Another significant benefit of earning a CRP certification is the potential for higher earnings. According to recent industry reports, certified research professionals often command higher salaries than their non-certified counterparts. This is largely due to the advanced level of knowledge and expertise that certified professionals bring to their roles.
Salary Advantages for CRP-Certified Professionals:
Higher Base Salaries: Clinical research professionals with a CRP certification tend to earn more than those without. The certification can lead to an average salary increase of 10% to 20% depending on the individual’s experience and the region in which they work.
Bonuses and Incentives: Many employers offer performance-based bonuses or incentives for CRP-certified professionals, particularly if they play a key role in the successful completion of clinical trials.
For example, a clinical research manager with CRP certification may earn a salary of around $100,000 per year in the United States, compared to $80,000 for a non-certified counterpart. This salary boost is not limited to managers; even entry-level roles like clinical research coordinators can see significant pay increases after obtaining certification.
For more information on how certification can impact your salary, you can check out this comprehensive Clinical Research Coordinator Certification Course.
3. Enhanced Knowledge and Skills
Earning a CRP certification requires candidates to undergo rigorous training and pass an examination that tests their understanding of clinical research processes, ethical guidelines, and regulatory requirements. This ensures that certified professionals are equipped with the latest knowledge and best practices in the field, which can help them excel in their roles.
Areas of Knowledge Gained:
Regulatory Compliance: CRP-certified professionals are well-versed in the rules and regulations governing clinical trials, including FDA guidelines, ICH-GCP standards, and international regulatory frameworks.
Ethical Considerations: Certified individuals are trained to ensure that clinical trials are conducted ethically, with patient safety and informed consent being top priorities.
Data Management and Reporting: CRP certification programs emphasize the importance of accurate data collection, storage, and reporting, ensuring that trials are conducted with integrity.
The CRP certification process fosters a comprehensive understanding of the complexities of clinical research. Professionals who earn this certification are better equipped to handle challenges that may arise during trials, such as managing protocol deviations or addressing safety concerns.
In addition, ongoing education is a key component of maintaining certification. CRP-certified professionals are required to engage in continuing education to stay up-to-date with the latest developments in clinical research, making them valuable assets to their employers.
4. Improved Job Satisfaction and Professional Confidence
Having a CRP certification can lead to greater job satisfaction by boosting confidence in your abilities as a clinical research professional. Knowing that you have met rigorous standards and gained a deep understanding of the field can make your work more fulfilling and provide you with a sense of accomplishment.
How Certification Enhances Job Satisfaction:
Confidence in Decision-Making: Certified professionals are more confident in their ability to make informed decisions during clinical trials, from protocol development to adverse event reporting.
Recognition in the Industry: CRP certification is recognized globally, which can open up opportunities to work on high-profile trials and collaborate with leading researchers and sponsors.
Personal Fulfillment: Many certified professionals report feeling more fulfilled in their roles, as they can see the direct impact of their work on advancing medical research and improving patient outcomes.
For professionals who are passionate about clinical research, the CRP certification provides a way to validate their expertise and feel more confident in their contributions to the field. This confidence often translates to improved job performance and career satisfaction.
5. Increased Employability in a Growing Field
The demand for clinical research professionals is growing rapidly due to the increasing complexity of clinical trials and the need for regulatory compliance in the development of new therapies. Having a CRP certification can increase your employability by making you stand out in a crowded job market.
Growing Demand for Certified Professionals:
Increased Clinical Trials: The number of clinical trials conducted globally is rising, leading to a greater need for qualified professionals to manage these trials.
Regulatory Scrutiny: As regulatory requirements for clinical trials become stricter, organizations are seeking certified professionals who understand the importance of compliance and ethical research practices.
Global Opportunities: CRP certification is recognized internationally, meaning that certified professionals can seek employment opportunities in different countries or with global organizations.
For professionals looking to secure a stable and fulfilling career in clinical research, earning a CRP certification is a smart investment. With the healthcare industry continually evolving, certified professionals will continue to be in high demand across a range of sectors, including pharmaceuticals, biotechnology, and academic research institutions.
Why CRP Certification is a Game-Changer for Clinical Research Professionals
Earning a CRP certification provides numerous benefits, from career advancement and increased earning potential to enhanced knowledge and job satisfaction. As the demand for skilled clinical research professionals continues to grow, obtaining certification can help you stay competitive in the job market and secure roles with greater responsibility and higher salaries.
Moreover, the knowledge and skills gained through the certification process will not only enhance your professional capabilities but also increase your confidence in managing clinical trials, ensuring that they are conducted ethically, efficiently, and in compliance with regulatory standards.
For those considering a career in clinical research or looking to advance their current role, the Advanced Clinical Research Project Manager Certification can be a valuable step forward. It is one of many certifications that can help propel your career to new heights and open doors to exciting opportunities in the ever-evolving field of clinical research.
Reference Links:
National Institutes of Health (NIH) - Clinical Research Resources
World Health Organization (WHO) - Clinical Trials
ClinicalTrials.gov - Find Clinical Trials
Relevant Course Links:
Clinical Trial Manager Salary: What to Expect in 2024
A career as a clinical trial manager (CTM) is both rewarding and demanding, with responsibilities that range from overseeing clinical trials to ensuring regulatory compliance and managing research staff. One of the most commonly asked questions for those considering or currently in this field is, "What is the average clinical trial manager salary?" Understanding the salary range for this role is crucial for career planning, negotiating job offers, and assessing career growth potential.
In this blog, we will explore the factors that influence clinical trial manager salaries, including experience, geographic location, and the type of employer. We will also provide insights into how the role of a clinical trial manager has evolved and what that means for salary expectations moving forward into 2024.
What Does a Clinical Trial Manager Do?
Before diving into salary figures, it's essential to understand the scope of a clinical trial manager's responsibilities. A CTM is responsible for overseeing clinical trials from start to finish, ensuring that all activities comply with regulatory standards, Good Clinical Practice (GCP), and the trial’s protocol. They manage everything from site selection and staff training to data collection and reporting.
Some of the core responsibilities include:
Protocol Development and Management: CTMs collaborate with investigators and sponsors to develop and refine trial protocols.
Regulatory Compliance: Ensuring that the trial meets FDA, EMA, and other relevant regulatory requirements.
Data Collection Oversight: Managing the collection and storage of trial data, ensuring its accuracy and integrity.
Team Management: Supervising research staff, including clinical research associates (CRAs) and site managers.
Budget Management: Overseeing the financial aspects of clinical trials, ensuring that they stay within the approved budget.
Given the high level of responsibility, clinical trial managers are compensated accordingly. However, several factors can influence their salary range.
Factors Influencing Clinical Trial Manager Salary
The salary of a clinical trial manager can vary significantly depending on a variety of factors. Below are some of the key influences on what you can expect to earn as a clinical trial manager in 2024.
1. Experience Level
Like many professions, experience plays a critical role in determining salary. Entry-level clinical trial managers typically start at a lower salary, while those with several years of experience and a strong track record in managing trials can command significantly higher pay.
Entry-Level (0-3 Years): The average salary for an entry-level clinical trial manager is typically between $70,000 and $85,000 per year. Entry-level CTMs often work under more experienced managers and gradually take on greater responsibilities as they gain experience.
Mid-Level (3-7 Years): As CTMs gain more experience, their salaries can increase to between $85,000 and $110,000 annually. At this stage, managers are expected to handle larger trials, take on leadership roles, and have a deeper understanding of regulatory guidelines and trial management.
Senior-Level (7+ Years): Senior clinical trial managers with extensive experience can earn between $110,000 and $150,000 or more. At this level, CTMs are often involved in high-level decision-making and may oversee multiple trials at once.
For clinical trial managers aiming to further their career and boost their earning potential, obtaining additional certifications, such as the Advanced Clinical Research Project Manager Certification, can be a smart move. Specialized knowledge in areas like project management or regulatory affairs can make CTMs more competitive in the job market and justify higher salaries.
2. Geographic Location
Geographic location is another key factor in determining salary. Clinical trial managers working in large metropolitan areas or regions with a high concentration of pharmaceutical companies or research institutions often earn more than those in smaller towns or rural areas.
United States: Clinical trial managers in the U.S. have some of the highest earning potentials globally. According to data from the Bureau of Labor Statistics, the average salary for CTMs in the U.S. is approximately $104,000 per year. However, this figure can be much higher in states like California, New York, and Massachusetts, where salaries can exceed $130,000 annually due to the high demand for clinical research professionals.
Europe: Salaries for clinical trial managers in Europe vary by country. In the United Kingdom, the average salary ranges between £50,000 and £70,000 annually. In Germany, CTMs earn between €60,000 and €90,000 per year. The demand for CTMs in these regions is also increasing, driven by growing investments in clinical research and biopharmaceuticals.
Asia-Pacific: The salary range for clinical trial managers in Asia-Pacific countries such as India and China tends to be lower than in Western countries, but the demand for qualified professionals is rising. In India, the average salary for a CTM is approximately ₹1,200,000 to ₹1,500,000 per year, while in China, it ranges between ¥300,000 and ¥600,000.
3. Type of Employer
The type of organization employing a clinical trial manager also significantly affects salary. Common employers of CTMs include pharmaceutical companies, contract research organizations (CROs), hospitals, and academic institutions.
Pharmaceutical Companies: CTMs working for large pharmaceutical companies typically earn the highest salaries, with averages ranging from $100,000 to $150,000 per year. These organizations often manage large-scale, multi-site clinical trials, and CTMs are expected to handle significant responsibilities.
Contract Research Organizations (CROs): Salaries at CROs are generally slightly lower than those in pharmaceutical companies, ranging from $85,000 to $120,000. However, working for a CRO can offer a broader range of experiences, as CTMs may manage trials across various therapeutic areas and sponsors.
Academic Institutions and Hospitals: CTMs employed by academic institutions or hospitals usually earn less than those in the private sector, with salaries ranging from $70,000 to $95,000. These positions may involve more specialized or investigator-initiated trials, which can be less financially lucrative but highly rewarding in terms of scientific impact.
Benefits and Additional Compensation
In addition to base salaries, clinical trial managers often receive additional forms of compensation, such as:
Bonuses: Many employers offer performance-based bonuses, especially for completing trials on time and within budget. These bonuses can range from 5% to 20% of the annual salary.
Stock Options or Profit Sharing: Some pharmaceutical companies offer stock options or profit-sharing programs as part of their compensation packages.
Comprehensive Benefits Packages: Full-time CTMs usually receive benefits such as health insurance, retirement plans, paid time off, and professional development opportunities.
It's important to consider these additional benefits when evaluating overall compensation, as they can significantly impact your total earnings.
How to Increase Your Salary as a Clinical Trial Manager
If you’re looking to boost your salary as a clinical trial manager, there are several strategies you can employ:
1. Pursue Advanced Certifications
As mentioned earlier, obtaining advanced certifications such as the Clinical Research Coordinator or Clinical Project Manager certifications can enhance your qualifications and make you a more attractive candidate for higher-paying positions.
2. Gain Specialized Experience
Working on high-profile clinical trials, particularly those involving new drug development or cutting-edge technologies, can enhance your resume and justify a higher salary. Experience in specific therapeutic areas, such as oncology or rare diseases, can also increase your value to employers.
3. Seek Opportunities in High-Paying Regions
If you are flexible with your location, consider seeking positions in regions with higher salary ranges, such as major metropolitan areas or countries where clinical research is in high demand.
4. Take on Leadership Roles
Moving into a senior or executive-level management position can significantly increase your earning potential. These roles often come with broader responsibilities, such as overseeing multiple clinical trials or managing entire research teams.
Salary Expectations for Clinical Trial Managers in 2024
The clinical trial manager salary varies based on several factors, including experience level, geographic location, and employer type. However, 2024 looks promising for professionals in this field, as demand for clinical trials continues to grow, especially in the wake of increased global investment in healthcare and drug development.
For clinical trial managers looking to maximize their earning potential, pursuing advanced certifications, gaining specialized experience, and seeking opportunities in high-demand regions are all effective strategies. As the field continues to evolve, so too will the opportunities for clinical trial managers to command competitive salaries while contributing to the advancement of medical science.
For those interested in further developing their skills and qualifications, consider enrolling in the Advanced Clinical Research Project Manager Certification to stay competitive in this dynamic field.
Reference Links:
University of California, San Diego – Clinical Research Career Development
Harvard University – Clinical Trials and Regulatory Requirements
U.S. Bureau of Labor Statistics – Medical and Health Services Managers
Relevant Course Links:
Study Start-Up Training Checklist for Research Staff
Clinical research is a vital part of advancing medical science, and research staff play a critical role in ensuring the success of clinical trials. The study start-up phase is one of the most complex and important stages in the clinical trial process. Proper training during this phase is essential to ensure that the trial runs smoothly, adheres to regulatory standards, and achieves its intended outcomes.
A well-structured study start-up training checklist for research staff ensures that all team members are prepared for the challenges of the study. This guide provides a comprehensive checklist to help research staff, clinical coordinators, and principal investigators organize and execute clinical trials efficiently. By following these steps, you can ensure that your clinical research team is well-prepared and the trial is set up for success.
What is Study Start-Up in Clinical Research?
Study start-up (SSU) is the phase that begins once the study sponsor has selected sites for the trial. This phase includes all the activities required to initiate a clinical trial at a specific site, such as gaining regulatory approvals, finalizing contracts, training staff, and setting up study documentation. The process is often complex, involving multiple stakeholders, including research staff, sponsors, regulatory bodies, and Institutional Review Boards (IRBs).
The effectiveness of the study start-up phase often determines the overall success of the clinical trial. A well-organized study start-up phase reduces delays, ensures compliance with regulatory requirements, and improves the chances of recruiting and retaining study participants.
Why is a Study Start-Up Training Checklist Important?
A training checklist is essential because it helps research staff stay organized and ensures that no critical steps are missed during the study start-up phase. Missing a crucial task or failing to properly train the staff can lead to delays, protocol deviations, or non-compliance with regulatory guidelines, which can ultimately jeopardize the entire clinical trial.
A checklist also ensures that all team members are on the same page, which improves communication and collaboration. By having a clear outline of the steps to be completed, research staff can focus on their specific tasks, resulting in greater efficiency and a smoother trial launch.
Key Components of a Study Start-Up Training Checklist
The checklist for training research staff during the study start-up phase should include several key components, ranging from regulatory preparation to logistical coordination. Below, we provide a detailed breakdown of these components.
1. Regulatory Documentation and Compliance
The first and foremost priority during the study start-up phase is ensuring that all regulatory documentation is in place. This includes, but is not limited to:
Institutional Review Board (IRB) Approval: Ensure that the study protocol has been reviewed and approved by the IRB or Ethics Committee. Without this approval, no study activities can proceed.
FDA and Other Regulatory Submissions: If applicable, submit the necessary documentation to the FDA or relevant regulatory authorities.
Informed Consent Forms (ICFs): Confirm that the informed consent forms are prepared and approved by the IRB. Research staff should be trained on the importance of obtaining proper informed consent from participants.
Internal Link: For more information on regulatory compliance in clinical trials, check out this Clinical Research Coordinator Certification Course.
2. Staff Training and Responsibilities
All members of the research team must receive thorough training to ensure they understand their roles and responsibilities. This includes:
Principal Investigator (PI) Training: The PI must be trained on the study protocol, the roles of other staff members, and the responsibilities they hold as the primary leader of the trial.
Study Coordinator Training: The clinical research coordinator is often responsible for the day-to-day operations of the trial. They need to be trained on subject recruitment, data collection, and maintaining study documentation.
Investigator Meeting Attendance: Ensure that all key research staff attend the investigator meeting, which is typically organized by the sponsor to discuss study procedures, safety protocols, and reporting requirements.
Additionally, research staff should receive Good Clinical Practice (GCP) training and be certified if they haven’t been already.
3. Protocol Training
Understanding the study protocol is essential for all research staff. This step ensures that everyone involved is familiar with the study design, eligibility criteria, intervention methods, and data collection processes. Key components of protocol training include:
Study Objectives and Hypotheses: Ensure that all staff understand the goals of the trial and the research questions it aims to answer.
Eligibility Criteria: Review the inclusion and exclusion criteria in detail to ensure proper participant recruitment.
Safety Monitoring and Adverse Event Reporting: Train staff on how to monitor for adverse events and report them to the appropriate regulatory bodies.
Research staff should regularly revisit the protocol throughout the study to ensure adherence to its guidelines.
4. Data Collection and Management
Accurate data collection is critical in clinical research. Research staff should be trained on:
Electronic Data Capture (EDC) Systems: Ensure that staff are proficient in using the EDC system for recording and managing study data.
Source Documentation: Train staff on maintaining accurate and compliant source documents. Source documents must provide a clear audit trail for all study-related activities.
Data Integrity and Confidentiality: Review best practices for maintaining data accuracy and patient confidentiality, in compliance with HIPAA or other relevant privacy regulations.
5. Site Preparation
Once regulatory and protocol training are complete, focus should shift to preparing the physical site for study activities. The checklist should include:
Clinical Supplies: Ensure that all necessary clinical supplies, such as lab kits, are available on-site.
Study Equipment: Verify that any specialized study equipment (e.g., diagnostic tools) is calibrated and functioning correctly.
Pharmacy Coordination: Work with the site pharmacy to ensure proper handling and storage of study drugs, if applicable.
6. Subject Recruitment and Enrollment
Recruitment is often one of the most challenging aspects of clinical research. Research staff should be trained on recruitment strategies, as well as the ethical considerations involved in subject recruitment. Key components include:
Recruitment Plan: Develop a recruitment plan based on the study’s target population and inclusion/exclusion criteria.
Informed Consent Process: Research staff must be proficient in explaining the study to potential participants and obtaining informed consent in accordance with regulatory guidelines.
Screening and Enrollment: Train staff to accurately screen potential participants for eligibility and document the screening process thoroughly.
7. Budget and Financial Management
The financial aspects of a clinical trial, including budgeting and payments to participants or investigators, should also be part of the study start-up training checklist. Research staff involved in financial management should:
Understand the Study Budget: Ensure that the trial’s budget is clearly understood and that all research staff are aware of their responsibilities in managing financial aspects.
Participant Compensation: Set up processes for compensating study participants in a timely and ethical manner.
Tracking Expenses: Implement a system for tracking all study-related expenses to ensure that the trial remains within budget.
Internal Monitoring and Auditing
Regular monitoring and internal auditing are essential to ensuring that the clinical trial is being conducted in compliance with the protocol and regulatory requirements. Research staff should:
Conduct Routine Audits: Ensure that all study documentation is up to date and that study activities are being conducted according to the protocol.
Address Protocol Deviations: Train staff on identifying and addressing any deviations from the study protocol.
Monitoring ensures that the trial stays on course and that any issues are addressed promptly before they escalate into significant problems.
Conclusion: A Well-Prepared Team Ensures Success
The study start-up training checklist for research staff is a vital tool in ensuring that clinical trials are conducted efficiently, ethically, and in compliance with regulatory standards. By following this checklist, research staff can be confident that they are well-prepared to execute a successful study. Proper training and preparation not only improve the chances of trial success but also protect participant safety and data integrity.
For more comprehensive training and certifications to enhance your clinical research career, check out this Advanced Clinical Research Project Manager Certification.
References:
National Institutes of Health. (2023). Clinical Trials: Overview and Process.
University of California, San Francisco. (2022). Ethics in Clinical Research.
This blog provides a structured approach to study start-up, ensuring that research staff are well-prepared to contribute to the successful execution of clinical trials.
Clinical Research Management System
Clinical research is a critical aspect of healthcare advancements, responsible for developing new treatments and medical technologies. The complexity of managing clinical trials, especially those involving multiple phases and diverse participants, has led to the development of Clinical Research Management Systems (CRMS). A CRMS is a software platform designed to facilitate the planning, tracking, and management of clinical trials, ensuring regulatory compliance and improving data accuracy.
In this blog, we’ll explore the core functions of a clinical research management system, its importance in the medical research industry, and how it can be leveraged to streamline clinical trials. We will also examine how a well-designed CRMS can improve efficiency, reduce costs, and maintain compliance across multiple regulatory bodies.
What is a Clinical Research Management System?
A Clinical Research Management System (CRMS) is a comprehensive software solution used by clinical researchers, project managers, and trial administrators to manage the various aspects of a clinical trial. These systems help organize, monitor, and report on the different activities involved in clinical research, such as subject recruitment, protocol adherence, and data management. They are crucial for the execution of both large-scale international trials and smaller, localized studies.
CRMS platforms are designed to be highly customizable, allowing organizations to tailor them according to the specific requirements of their clinical studies. With features that range from patient enrollment and informed consent to data analysis and regulatory reporting, a well-implemented CRMS plays a vital role in ensuring the integrity and success of clinical trials.
Key Features of a Clinical Research Management System
A robust CRMS offers a wide array of features that cater to the needs of clinical researchers, sponsors, and regulatory bodies. Some of the key components include:
1. Study Design and Protocol Management
This feature enables research teams to define the structure of their clinical trials, including the phases, study sites, participant groups, and protocols. It ensures that all trial activities are aligned with the approved study plan and regulatory requirements.
2. Subject Recruitment and Tracking
Managing patient recruitment is a critical task in clinical trials. CRMS platforms streamline the recruitment process by identifying eligible candidates, tracking their progress through the trial phases, and documenting their informed consent. Efficient recruitment is essential for maintaining timelines and ensuring the study population reflects the desired demographic.
3. Data Management and Integrity
Clinical trials generate vast amounts of data. A CRMS ensures that data is collected, stored, and managed in compliance with Good Clinical Practice (GCP) guidelines. It facilitates the secure sharing of data between research teams, sponsors, and regulatory bodies, while ensuring that data integrity is maintained throughout the trial.
4. Regulatory Compliance
Adherence to regulatory standards such as FDA, EMA, and ICH-GCP is critical in clinical research. A CRMS is designed to ensure that all trial activities are compliant with these standards. It helps streamline the audit process and simplifies the submission of reports to regulatory authorities. This not only ensures compliance but also reduces the risk of costly delays or trial terminations.
5. Financial and Budget Management
Clinical trials are expensive, and managing the financial aspects can be challenging. CRMS platforms include financial tracking tools to monitor expenses, manage contracts, and handle payments to investigators and participants. This feature ensures that the trial remains within budget and that financial reporting is transparent and accurate.
6. Real-Time Reporting and Analytics
The ability to generate real-time reports and analytics is one of the most valuable features of a CRMS. These insights help researchers make informed decisions about trial progression, identify any deviations from protocols, and optimize resource allocation. Having access to real-time data also facilitates quicker decision-making, which can improve the overall efficiency of the trial.
Why is a Clinical Research Management System Important?
The complexity and scale of modern clinical trials necessitate the use of sophisticated tools like CRMS to manage and coordinate all activities efficiently. Here are a few reasons why implementing a CRMS is crucial for clinical research organizations:
1. Improved Efficiency
A CRMS automates many of the labor-intensive tasks involved in trial management, such as data entry, patient tracking, and regulatory reporting. This automation reduces the burden on research staff, freeing them to focus on more critical aspects of the trial, such as patient care and protocol adherence.
2. Enhanced Data Accuracy and Integrity
Human error is a significant risk in clinical research, especially when managing large datasets. A CRMS ensures that data is captured accurately and stored securely, minimizing the risk of errors and improving data integrity. Additionally, CRMS platforms offer audit trails, making it easier to trace any changes made to the data throughout the trial.
3. Streamlined Regulatory Compliance
Meeting regulatory standards is one of the most challenging aspects of clinical research. A CRMS is designed to ensure that all trial activities adhere to the necessary regulations, reducing the risk of non-compliance. By integrating regulatory requirements into the workflow, the system helps organizations avoid costly mistakes and delays.
4. Cost Reduction
The automation and streamlined processes provided by a CRMS can significantly reduce the costs associated with clinical trials. By improving resource allocation, reducing manual data entry, and minimizing protocol deviations, a CRMS helps organizations conduct trials more cost-effectively.
5. Increased Transparency and Accountability
A CRMS enhances transparency by providing stakeholders with real-time access to trial data, progress reports, and financial statements. This transparency fosters accountability and ensures that all parties involved in the trial are aligned with its goals and timelines.
Integrating CRMS with Other Systems
In today’s clinical research environment, CRMS platforms often need to integrate with other systems to provide a comprehensive solution for managing clinical trials. Common integrations include:
Electronic Data Capture (EDC) systems: Used to collect and manage clinical data.
Clinical Trial Management Systems (CTMS): Oversee the operational aspects of trials, such as site management and resource allocation.
Regulatory Information Management Systems (RIMS): Manage regulatory submissions and track approvals.
By integrating these systems, a CRMS provides a centralized platform that enhances collaboration between research teams, sponsors, and regulatory bodies.
Best Practices for Implementing a Clinical Research Management System
To maximize the benefits of a CRMS, it’s essential to follow best practices during implementation. Here are some key steps to ensure a successful deployment:
1. Assess Organizational Needs
Before selecting a CRMS, it’s important to assess your organization’s specific needs. Consider the size and scope of your clinical trials, regulatory requirements, and budget constraints. This assessment will help you choose a system that aligns with your goals.
2. Ensure User Training
Implementing a CRMS requires buy-in from all stakeholders, including researchers, administrators, and sponsors. Providing comprehensive training ensures that all users are familiar with the system’s features and can use it effectively.
3. Maintain Data Security
Clinical trials involve sensitive patient information, so it’s crucial to prioritize data security when implementing a CRMS. Ensure that the system complies with data protection regulations such as HIPAA and GDPR, and use encryption and access controls to safeguard patient data.
4. Monitor and Optimize
After implementing the CRMS, it’s important to continuously monitor its performance and optimize its use. Regular audits and feedback from users can help identify areas for improvement and ensure that the system continues to meet the needs of your organization.
Conclusion: The Future of Clinical Research Management Systems
The increasing complexity of clinical trials and the growing need for regulatory compliance have made CRMS platforms indispensable tools in the medical research industry. By automating key processes, improving data integrity, and streamlining regulatory reporting, a well-designed CRMS can significantly enhance the efficiency and success of clinical trials. As clinical research continues to evolve, so too will the capabilities of CRMS platforms, offering even greater opportunities for improving patient outcomes and advancing medical science.
For more information on clinical trial management and regulatory compliance, check out this course on Clinical Trials Assistant Training.
Reference Links:
National Institutes of Health (NIH) – Clinical Trials Overview
ClinicalTrials.gov – A Service of the U.S. National Library of Medicine
European Medicines Agency (EMA) – Clinical Trials Regulation
Relevant Course Links:
Acronym of a Title in Clinical Research: Understanding the Key Terminology
In the world of clinical research, acronyms play an essential role in simplifying complex medical terms and protocols. Whether you're new to the field or a seasoned professional, mastering these acronyms is crucial for seamless communication and understanding across teams, departments, and institutions. This blog aims to shed light on the most commonly used acronyms in clinical research, their significance, and how they contribute to the efficiency of clinical trials and studies.
What Is an Acronym in Clinical Research?
An acronym is a shorthand term formed from the first letters of a series of words. In clinical research, acronyms are widely used to represent important processes, roles, and regulations. These acronyms help researchers, medical professionals, and regulatory authorities communicate more efficiently, especially when dealing with lengthy medical terms. For example, ICH-GCP (International Conference on Harmonisation – Good Clinical Practice) is a globally recognized acronym that sets ethical and quality standards for conducting clinical trials.
Acronyms are not only used in written documents but also in verbal communication, streamlining discussions about complex clinical topics. From understanding regulatory guidelines to identifying specific roles within a clinical trial, acronyms simplify the dialogue and foster clarity.
Why Are Acronyms Important in Clinical Research?
The clinical research field involves an extensive amount of data collection, regulatory compliance, and scientific study. This often results in the use of highly technical jargon, which can be cumbersome. Using acronyms helps reduce the complexity of communication and enhances understanding between various stakeholders, such as principal investigators, clinical research coordinators, and regulatory authorities.
Here are some reasons why acronyms are pivotal in clinical research:
Efficiency in Communication: Acronyms condense long phrases into shorter, more manageable forms.
Standardization Across the Industry: Many acronyms, such as GCP (Good Clinical Practice) and CRA (Clinical Research Associate), are universally accepted, ensuring consistency across institutions and borders.
Time-Saving: Reducing the length of documents and conversations allows for quicker decision-making and faster communication between teams.
Improved Understanding: Even those new to clinical research can quickly familiarize themselves with commonly used acronyms, easing their integration into the field.
Common Acronyms in Clinical Research
ICH-GCP (International Conference on Harmonisation - Good Clinical Practice)
Purpose: ICH-GCP is a set of internationally accepted guidelines that ensure the ethical and scientific quality of clinical trials. These guidelines protect the rights and safety of trial participants and ensure that clinical data is credible and accurate.
Usage: ICH-GCP guidelines are mandatory for clinical trials involving human participants, and professionals in clinical research must be well-versed in these standards.
Learn More: Advanced Principal Investigator Physician Certification
FDA (Food and Drug Administration)
Purpose: The FDA is a regulatory body in the United States that ensures the safety and efficacy of drugs, medical devices, and biological products. It plays a critical role in the approval process for new medications and treatments.
Usage: The FDA oversees clinical trials to ensure they comply with safety regulations, ethical guidelines, and quality standards.
Learn More: Clinical Research Coordinator Training
CRO (Contract Research Organization)
Purpose: A CRO is an external organization hired by pharmaceutical companies or medical institutions to manage clinical trials. They provide expertise and resources to ensure that studies are conducted efficiently and in compliance with regulatory standards.
Usage: CROs are often involved in all phases of clinical research, from protocol development to data analysis.
Learn More: ICH-GCP Training
IRB (Institutional Review Board)
Purpose: The IRB is a group that reviews and approves the ethical aspects of clinical trials to ensure the safety and rights of participants are protected.
Usage: All clinical trials involving human participants must receive IRB approval before beginning the study.
Learn More: Clinical Trials Assistant Training
AE/SAE (Adverse Event/Serious Adverse Event)
Purpose: An AE refers to any unfavorable medical occurrence in a participant during a clinical trial, while an SAE is a life-threatening event or one that results in death, hospitalization, or disability.
Usage: Reporting of AE/SAEs is critical for patient safety and must be documented meticulously in compliance with regulatory standards.
The Role of Acronyms in Regulatory and Ethical Compliance
Acronyms such as IRB, GCP, and FDA are not merely abbreviations but represent essential entities that maintain the integrity of clinical research. Regulatory compliance, ethical approvals, and safety reporting are critical areas where acronyms ensure that important guidelines are followed strictly.
For example, GCP governs the ethical conduct of trials to ensure that human participants' rights are prioritized and that the results are scientifically valid. Similarly, IRBs play a pivotal role in reviewing clinical trial protocols to ensure that ethical standards are met. These acronyms may represent complex systems, but their usage is straightforward, allowing professionals to focus on their core work without constantly revisiting lengthy documentation.
Tips for Understanding Acronyms in Clinical Research
Mastering the acronyms used in clinical research requires time and practice. Below are some helpful tips for professionals looking to improve their understanding of clinical research acronyms:
Create a Glossary: Keeping a personal glossary of the most frequently used acronyms will help you become more familiar with them over time.
Use Flashcards: Flashcards can be an effective way to memorize acronyms and their full forms, especially for those new to clinical research.
Stay Updated: Clinical research is a dynamic field, and new acronyms are introduced as the industry evolves. Stay up-to-date by reading industry publications and attending relevant conferences.
The Future of Acronyms in Clinical Research
As clinical research continues to evolve with advancements in technology and regulations, the use of acronyms will remain integral to the industry. New acronyms are constantly being introduced to describe emerging technologies, regulatory updates, and novel therapeutic approaches. For example, the rise of AI (Artificial Intelligence) and ML (Machine Learning) in clinical trials has led to new terminology that is now becoming part of the clinical research lexicon.
Moreover, as global collaboration in clinical research grows, acronyms will help bridge language barriers and foster better communication among international teams. Acronyms like GCP are already universally recognized and will continue to play a vital role in standardizing clinical research practices across the world.
Learn More
For those interested in deepening their knowledge of clinical research, understanding the role of these acronyms can be a stepping stone to more advanced training. You can explore these resources to gain further insights into the industry:
These courses provide comprehensive information on clinical research practices and are ideal for professionals looking to elevate their expertise.
Wrapping Up
Acronyms in clinical research are far more than just shorthand. They represent vital processes, roles, and regulatory guidelines that ensure the ethical, efficient, and scientific conduct of clinical trials. Whether you're a clinical research professional or new to the field, understanding and using these acronyms correctly will enhance your communication skills and deepen your knowledge of this intricate industry.
By familiarizing yourself with the key acronyms discussed in this blog, you’ll be well on your way to becoming more proficient in navigating clinical research.
To continue expanding your expertise in clinical research, consider taking specialized courses that delve into the acronyms and terminology of this fascinating field. For example, you can explore more about the ICH-GCP guidelines or pursue certifications in areas like clinical research project management or pharmacovigilance, both of which heavily rely on understanding clinical research acronyms.
Reference Links:
National Institutes of Health (NIH) – Clinical Research Training
U.S. Food and Drug Administration (FDA) – Clinical Trials Overview
Relevant Course Links:
How to Become a Clinical Project Manager
Becoming a clinical project manager (CPM) is a fulfilling career path that offers the opportunity to lead and manage clinical trials, ensuring that research is conducted smoothly and in accordance with regulatory standards. Clinical project managers are vital in the healthcare industry, as they help bring new medical innovations to the market. If you are considering this career, here’s a detailed guide on the steps you need to take, the qualifications required, and the skills you will need to succeed in this role.
Step 1: Obtain a Relevant Bachelor's Degree
The first step toward becoming a clinical project manager is earning a bachelor’s degree in a relevant field. While there isn’t a specific degree titled “Clinical Project Management,” degrees in life sciences, health sciences, biology, chemistry, or pharmacology are common paths. A background in these areas provides the foundational knowledge necessary for understanding clinical trials and the drug development process.
Popular Undergraduate Degrees for Clinical Project Managers:
Biology: Offers a deep understanding of human biology and laboratory practices.
Chemistry: Focuses on drug formulation and chemical processes.
Nursing: Provides insight into patient care and clinical settings.
Public Health: Covers broader health issues and epidemiology, which are relevant to clinical trials.
Additionally, some students pursue degrees in business administration or project management to strengthen their understanding of managing complex projects.
Step 2: Gain Experience in Clinical Research
A crucial step in becoming a clinical project manager is gaining experience in the clinical research field. Many start their careers in entry-level roles such as clinical research coordinators (CRCs), clinical trial assistants (CTAs), or clinical research associates (CRAs). These positions allow you to learn the ins and outs of clinical trials, from patient recruitment to data collection and regulatory compliance.
Common Entry-Level Roles:
Clinical Research Coordinator (CRC): Coordinates the daily operations of clinical trials.
Clinical Trial Assistant (CTA): Provides administrative support to project teams.
Clinical Research Associate (CRA): Monitors trial sites to ensure compliance with protocols and regulations.
Working in these roles helps you understand the regulatory framework and operational challenges that clinical project managers will eventually oversee. Experience in these positions also offers valuable insights into patient management, protocol adherence, and communication with study stakeholders.
For those looking to jumpstart their career, enrolling in a specialized course like the Clinical Research Coordinator training or the Clinical Trials Assistant Training course can give you the necessary skills and certification to enter the field.
Step 3: Earn Certifications and Advanced Training
While experience in clinical research is important, certifications are increasingly sought after by employers looking to hire clinical project managers. Earning certifications in clinical research management demonstrates your commitment to professional development and validates your expertise.
Some of the most recognized certifications include:
Project Management Professional (PMP): Offered by the Project Management Institute (PMI), this certification covers general project management practices applicable to all industries, including clinical trials.
Certified Clinical Research Professional (CCRP): Provided by the Society of Clinical Research Associates (SOCRA), this certification is specifically focused on clinical research professionals.
Certified Clinical Project Manager (CCPM): This is a specialized certification that focuses on the unique responsibilities and challenges of managing clinical trials.
To gain a deeper understanding of the regulatory and operational aspects of clinical trials, consider enrolling in advanced courses such as the Advanced Clinical Research Project Manager Certification or CRA training, which will equip you with the necessary skills to excel in this role.
Step 4: Develop Essential Skills for Clinical Project Management
Successful clinical project managers possess a unique combination of scientific knowledge, leadership abilities, and organizational skills. Here are some of the key skills required:
Leadership and Team Management:
CPMs oversee multidisciplinary teams, including scientists, clinicians, and regulatory experts. Strong leadership skills are needed to motivate, coordinate, and manage these diverse teams.
Communication:
CPMs must be excellent communicators, as they often serve as a liaison between research teams, sponsors, and regulatory authorities. Effective communication ensures that all stakeholders are informed and aligned on the trial’s progress.
Budgeting and Financial Management:
Managing the financial aspects of clinical trials, including budgeting and resource allocation, is a critical responsibility. CPMs must ensure that the project stays within budget without compromising the quality of the research.
Regulatory Knowledge:
Clinical trials are governed by strict regulations, including the International Council for Harmonisation (ICH) guidelines and Good Clinical Practice (GCP) standards. CPMs must stay up-to-date with these regulations to ensure compliance throughout the trial.
Problem-Solving and Risk Management:
Clinical trials often encounter unforeseen challenges, such as patient recruitment delays or data inconsistencies. CPMs must be skilled in identifying potential risks early on and developing strategies to mitigate them.
Data Analysis and Interpretation:
While not directly responsible for analyzing trial data, CPMs must have a thorough understanding of how clinical data is collected and interpreted to ensure that study results are accurate and reliable.
Step 5: Gain Experience in Project Management
In addition to clinical research experience, it is essential to gain hands-on experience in managing projects. This can be done by taking on leadership roles within clinical trials or managing smaller projects under the guidance of an experienced clinical project manager.
Many clinical project managers start in roles such as associate project managers, where they learn the skills needed to oversee timelines, budgets, and team coordination. This experience prepares them for the more complex responsibilities of leading entire clinical trials.
Step 6: Pursue a Master’s Degree (Optional but Beneficial)
While a bachelor’s degree is sufficient for entry-level roles, a master’s degree in clinical research, project management, or business administration can significantly enhance your prospects of becoming a clinical project manager. Graduate programs provide advanced knowledge in research methodologies, project management strategies, and regulatory affairs, equipping you for higher-level positions.
Some institutions offer specialized master's degrees in clinical research management or healthcare project management. These programs are designed to provide the skills needed to oversee clinical trials effectively and prepare for leadership roles in the field.
Step 7: Network and Stay Current with Industry Trends
The healthcare and clinical research industries are constantly evolving, with new regulations, technologies, and methodologies emerging regularly. Staying informed about these changes is crucial for clinical project managers. Attending industry conferences, webinars, and continuing education courses can help you stay updated on the latest trends.
Networking is equally important. Building relationships with other professionals in the field can provide opportunities for career advancement, mentorship, and collaborative projects. Consider joining professional organizations such as the Association of Clinical Research Professionals (ACRP) or the Society of Clinical Research Associates (SOCRA) to stay connected with the latest developments in the industry.
Career Outlook and Opportunities for Clinical Project Managers
The demand for clinical project managers is growing due to the increasing number of clinical trials worldwide, particularly in areas like oncology, cardiology, and neurology. According to the U.S. Bureau of Labor Statistics, employment in clinical research is expected to grow faster than the average for all occupations over the next decade.
Clinical project managers can work for:
Contract Research Organizations (CROs): These organizations are often contracted by pharmaceutical companies to manage clinical trials.
Pharmaceutical and Biotech Companies: Clinical project managers may work directly for drug development companies to manage in-house trials.
Academic and Research Institutions: Universities and research centers also conduct clinical trials and require CPMs to manage them.
Final Thoughts
Becoming a clinical project manager requires a blend of education, experience, and specialized skills. While it is a challenging role, it is also incredibly rewarding, offering the chance to contribute to cutting-edge medical research that can improve patient outcomes worldwide.
If you are interested in advancing your career in clinical project management, consider exploring advanced certifications and training programs, such as the Advanced Clinical Research Project Manager Certification, to give yourself a competitive edge.
By following these steps, you can build a successful career in clinical project management and play a crucial role in the future of healthcare innovation.
Reference Links:
U.S. National Library of Medicine – Clinical Research Careers
Harvard T.H. Chan School of Public Health – Master's in Clinical Research
Project Management Institute (PMI) – Project Management Professional (PMP) Certification
Society of Clinical Research Associates (SOCRA) – Certification Programs
Relevant Course Links:
Clinical Trials New Protocol Training Checklist for Study Nurses
In clinical trials, the role of study nurses is crucial. They are the backbone of the clinical trial team, ensuring that protocols are followed, patient safety is prioritized, and data is collected accurately. With the rise of more complex and diverse clinical trials, such as decentralized trials and adaptive trial designs, study nurses need to be thoroughly trained on new protocols before the study begins. A well-structured and comprehensive training checklist ensures that study nurses are fully equipped to handle the specific requirements of each clinical trial.
This blog provides a clinical trials new protocol training checklist for study nurses, helping them navigate the intricacies of clinical trials with confidence and precision. This checklist is essential for ensuring compliance with Good Clinical Practice (GCP) standards, regulatory requirements, and the study’s specific protocols.
Why Protocol Training is Essential for Study Nurses
Before delving into the specifics of the training checklist, it is important to understand the significance of protocol training for study nurses. Protocols dictate every aspect of a clinical trial, from participant recruitment to data collection, and any deviation from these protocols can result in regulatory issues, safety concerns, or the invalidation of data. For study nurses, thorough understanding and adherence to these protocols are paramount.
Study nurses also act as the primary point of contact for trial participants. They must communicate effectively, ensure patient adherence to the protocol, and manage any adverse events that arise. Therefore, comprehensive protocol training not only helps study nurses maintain compliance but also improves the overall quality of the clinical trial.
Key Components of a New Protocol Training Checklist
A comprehensive checklist for protocol training ensures that study nurses receive a systematic introduction to all necessary trial components. This training typically covers clinical trial regulations, patient care, data collection, and specific trial procedures. Below is a detailed training checklist for study nurses involved in clinical trials:
1. Understanding the Protocol Overview
Study Objectives and Endpoints: Ensure that study nurses understand the trial’s primary and secondary objectives, as well as the key endpoints that need to be measured.
Study Design: Provide an overview of the study design, including randomization, blinding, and any special methodologies, such as adaptive designs or decentralized components.
Trial Phases: Ensure that nurses are familiar with the trial phase they will be working on, as procedures and requirements vary significantly between Phase I and Phase III trials.
2. Regulatory and Ethical Considerations
Good Clinical Practice (GCP): Study nurses must be trained in GCP standards to ensure ethical conduct and compliance with international regulations.
Institutional Review Board (IRB) Approvals: Make sure study nurses understand the IRB’s role in approving the protocol and any necessary amendments.
Informed Consent Process: Ensure that nurses are trained on the process of obtaining informed consent, including how to explain the trial’s risks and benefits to participants.
Patient Confidentiality: Nurses must be well-versed in maintaining patient confidentiality, particularly in decentralized trials where remote data collection may be involved.
3. Patient Recruitment and Eligibility
Inclusion/Exclusion Criteria: Nurses should fully understand the study’s inclusion and exclusion criteria, ensuring that only eligible participants are enrolled.
Screening Procedures: Provide training on patient screening, including any required diagnostic tests or medical histories.
Recruitment Strategies: Familiarize nurses with the trial’s recruitment plan, which may involve community outreach, advertising, or working with other healthcare providers.
4. Training on Study-Specific Procedures
Medication Administration: If the protocol involves investigational products, nurses must be trained on the correct administration methods, dosage schedules, and monitoring for adverse reactions.
Data Collection Tools: Train nurses on the use of Electronic Data Capture (EDC) systems, ensuring they know how to input data accurately and in compliance with the protocol.
Monitoring and Reporting Adverse Events: Nurses should be trained on identifying and reporting adverse events (AEs) and serious adverse events (SAEs), including the use of safety reporting systems.
5. Decentralized and Remote Trial Components
Telehealth Training: For decentralized trials, nurses must be familiar with telehealth platforms for remote consultations and follow-ups.
Mobile Health Technology: Train nurses on using mobile health apps and devices, such as wearables, to monitor patient data remotely.
Remote Monitoring and Data Security: Ensure that nurses understand the protocols for remote monitoring of patient data and are aware of security measures to protect sensitive patient information.
6. Patient Management and Care
Patient Communication: Equip nurses with communication strategies to improve patient adherence and retention, ensuring patients understand their responsibilities during the trial.
Follow-Up Procedures: Outline the follow-up schedule and procedures for study participants, whether conducted in person or remotely.
Managing Side Effects: Nurses should be prepared to educate participants about potential side effects of the investigational product and how to manage them.
7. Training on Data Integrity and Documentation
Source Data Verification: Nurses should be trained on how to ensure data accuracy and integrity, including source data verification procedures.
Electronic Health Records (EHR) Integration: In some trials, data from EHRs may be integrated with the trial’s data collection system. Train nurses on the process of data extraction and integration.
Data Audits and Inspections: Provide guidance on how to prepare for potential audits or inspections by regulatory authorities, including maintaining accurate and complete documentation.
8. Trial-Specific Logistics
Study Drug Storage and Handling: If applicable, train nurses on the proper storage, handling, and documentation of study drugs, including any temperature monitoring requirements.
Supply Management: Nurses should understand how to manage trial supplies, including investigational products, test kits, and patient materials.
Patient Travel and Accommodation: In certain cases, nurses may need to assist with logistics related to patient travel or accommodations, particularly in multicenter or global trials.
Free Protocol Training Resources for Study Nurses
Many resources are available to help study nurses gain the knowledge and skills they need to manage clinical trials effectively. Below are some highly recommended free resources:
NIH Clinical Research Training: The National Institutes of Health (NIH) offer a variety of free online courses related to clinical research, including modules focused on Good Clinical Practice and regulatory requirements.
FDA Clinical Trials Training: The U.S. Food and Drug Administration (FDA) provides training resources that are particularly useful for study nurses involved in drug trials. These resources cover everything from protocol design to reporting adverse events.
For those looking to gain a deeper understanding of clinical project management and study protocol design, the Clinical Research Coordinator Certification offered by CCRPS is an excellent resource. This program provides comprehensive training for clinical research professionals, including study nurses, helping them navigate the complex landscape of clinical trials. Learn more about the course here.
Best Practices for Implementing a Protocol Training Checklist
Once you have a protocol training checklist in place, the next step is to implement it effectively. Here are some best practices for ensuring successful implementation:
Customize the Checklist for Each Study: While the core elements of protocol training may remain the same, it’s important to tailor the checklist to the specific requirements of each trial.
Use E-Learning Tools: Online learning platforms can be a great way to deliver protocol training, especially for decentralized teams. Consider using e-learning modules, quizzes, and simulations to engage study nurses and ensure they understand the material.
Provide Ongoing Training: Clinical trial protocols can change over time, and nurses may need to receive additional training if amendments are made. Make ongoing training a part of your clinical trial workflow to ensure compliance.
Document Training Completion: Maintain records of all completed training sessions, ensuring that study nurses have completed the necessary training before the trial begins. This documentation can be critical for audits and inspections.
Future Trends in Clinical Trial Protocol Training
As clinical trials continue to evolve, so too will the training requirements for study nurses. With the increasing adoption of digital health tools and decentralized trial models, nurses will need to be trained on the use of new technologies, such as telemedicine platforms, wearable devices, and AI-based data analysis tools.
Moreover, as the regulatory landscape changes, study nurses will need to stay updated on new guidelines from bodies like the FDA, EMA, and local regulatory authorities. Continuous professional development and access to new training materials will be essential.
Key Takeaways
A comprehensive clinical trials new protocol training checklist for study nurses is essential for ensuring the successful execution of any clinical trial. By following a structured checklist, study nurses can ensure they are fully prepared to handle the demands of modern clinical trials, whether decentralized, adaptive, or traditional. With the right training in place, nurses can maintain protocol compliance, improve patient outcomes, and ensure the integrity of trial data.
For those looking to further their knowledge and expertise, CCRPS offers a range of certification courses tailored to clinical research professionals, including nurses. You can explore these courses and learn more about improving your clinical trial skills here.
Reference Links:
National Institutes of Health (NIH) – Clinical Research Training
European Medicines Agency (EMA) – Clinical Trials Information System
Relevant Course Links:
Clinical Research Associate Salary Entry Level
Clinical research is a fast-growing field, with many aspiring professionals seeking a career as a Clinical Research Associate (CRA). These professionals play a pivotal role in the development of new treatments and medical advancements by ensuring that clinical trials adhere to regulatory guidelines, ethical standards, and research protocols. As with any career, one of the most common questions for newcomers is, "What can I expect in terms of salary as an entry-level Clinical Research Associate?"
In this blog, we will dive into the factors that influence a CRA's entry-level salary, provide a breakdown of potential earnings, and explore what professionals can expect as they gain experience. This guide is tailored to those just beginning their career in clinical research, helping you understand the financial rewards of this exciting and impactful profession.
What is a Clinical Research Associate (CRA)?
Before delving into salary specifics, it's important to have a clear understanding of the role. A Clinical Research Associate is responsible for overseeing clinical trials that test new drugs, treatments, and medical devices. Their duties often include:
Monitoring trial sites to ensure compliance with study protocols
Ensuring that data collection is accurate and complete
Verifying that the rights and safety of participants are protected
Liaising with medical professionals, sponsors, and regulatory bodies
This is a highly detail-oriented job that requires knowledge of Good Clinical Practice (GCP), clinical trial phases, and the regulatory landscape of the medical research industry. CRAs work with research teams and sponsors to ensure that trials run smoothly and safely.
Average Entry-Level Clinical Research Associate Salary
Global Averages
Entry-level Clinical Research Associate salaries can vary depending on location, company size, and the scope of the trial. In the United States, for example, the salary for a newly-hired CRA typically ranges from $50,000 to $70,000 annually. On the lower end, this salary reflects positions in smaller markets or with companies that offer limited research projects. In larger metropolitan areas, salaries can be on the higher end of this range due to the demand for CRAs and the complexity of projects.
Regional Variations
United States: As previously mentioned, the typical range for entry-level CRA salaries is $50,000 to $70,000. Those with specialized skills, such as expertise in regulatory affairs or a specific type of clinical trial (e.g., oncology), may command higher salaries even at the start of their careers.
Europe: In countries like the UK, Germany, and France, entry-level salaries range from €40,000 to €55,000 annually. The cost of living and demand for medical research professionals significantly impact salary figures.
Asia: In regions such as India and Southeast Asia, entry-level CRA salaries are often lower due to the differing economic conditions. In India, for example, entry-level CRAs can expect an average salary of ₹4 to ₹6 lakhs per year.
Company Size and Trial Complexity
Salaries also fluctuate depending on whether a CRA works for a small biotech firm, a large pharmaceutical company, or a contract research organization (CRO). Smaller firms may offer lower starting salaries but provide rapid career advancement opportunities. Conversely, large pharmaceutical companies and CROs offer competitive salaries and structured career paths, but promotion may take longer due to organizational size.
Additionally, the complexity of clinical trials plays a role. CRAs involved in global multi-center trials, especially those in high-demand areas like oncology or rare diseases, may receive a higher starting salary than those in more straightforward studies.
Key Factors Influencing Entry-Level CRA Salaries
1. Educational Background
One of the most critical factors in determining an entry-level CRA’s salary is educational qualifications. While a bachelor's degree in life sciences is generally the minimum requirement, candidates with advanced degrees such as a Master's in Clinical Research or relevant certifications can command higher starting salaries. Graduates from top-tier institutions or those with specialized research skills are more likely to receive competitive offers.
Some relevant certifications for aspiring CRAs include:
Certified Clinical Research Professional (CCRP)
Certified Clinical Research Coordinator (CCRC)
Advanced Clinical Medical Scribe Certification Course
Having a certification showcases a higher level of expertise and can position you for quicker salary growth.
2. Geographic Location
Location is another significant factor. Clinical research is often concentrated in major metropolitan hubs such as New York, Los Angeles, London, and Berlin. CRAs in these areas can expect higher salaries due to increased demand and a higher cost of living. In contrast, entry-level salaries in smaller cities or rural areas may be lower but come with the benefit of reduced competition for roles.
Additionally, international clinical research organizations may pay different rates depending on the region where the research is conducted, and many professionals find that relocating to a high-demand area can significantly boost their earning potential.
3. Industry Sector
There is a clear distinction between the salaries offered in biotech, pharmaceuticals, and medical devices. Pharmaceutical companies often lead in salary offers, followed by biotech firms. CROs, which specialize in managing clinical trials for various sponsors, typically offer competitive salaries but may be lower than in-house positions at pharmaceutical giants.
Pharmaceutical Companies: Often offer entry-level salaries on the higher end of the scale, between $60,000 to $75,000, due to the complexity and volume of trials they oversee.
Biotechnology Firms: Salaries are competitive, typically ranging from $55,000 to $70,000.
Contract Research Organizations (CROs): These organizations provide a range of salaries depending on their size and location but typically fall between $50,000 and $65,000 for entry-level roles.
4. Experience and Internships
While this blog is focused on entry-level salaries, it is important to note that internships or previous experience in clinical trials can influence salary negotiations. Candidates who have completed internships in clinical research, data management, or regulatory affairs may start with salaries on the higher end of the scale.
Employers highly value candidates who have had exposure to clinical trials, even at the entry level, because it reduces training time and increases the likelihood of the new hire succeeding in the role.
5. Soft Skills and Certifications
Beyond technical knowledge, CRAs must possess a range of soft skills that help them excel in their role. Excellent communication, problem-solving, time management, and an ability to work under pressure are qualities that often distinguish successful CRAs.
Candidates who demonstrate these skills during interviews, along with a certification in clinical research, often receive higher offers.
6. Demand for CRAs in Specific Therapeutic Areas
Not all CRAs are created equal, especially in entry-level roles. Clinical research in oncology, neurology, cardiology, and rare diseases commands higher salaries due to the complexity and high-stakes nature of these fields. If you're starting out and you’re able to specialize in one of these high-demand therapeutic areas, you may see a bump in your starting salary.
Salary Growth Over Time
Entry-level CRAs can expect salary growth as they gain more experience and take on more responsibilities. Within a few years, CRAs typically transition to Senior CRA roles or Clinical Trial Managers, with salaries ranging from $80,000 to $100,000 or more, depending on location and industry.
Additionally, the opportunity to move into regulatory affairs, project management, or business development opens up as CRAs gain a deeper understanding of clinical research operations. This career progression leads to further salary increases.
Conclusion
Becoming a Clinical Research Associate offers not only an exciting career in the development of medical treatments but also the potential for a rewarding salary. Entry-level CRAs can expect to earn between $50,000 and $70,000 in the U.S., with global variations influenced by geographic location, industry sector, and specific expertise. Factors such as educational background, internships, and specialization in high-demand areas can all impact starting salaries. For those entering this field, the potential for salary growth and career advancement is substantial, making the role of a CRA a lucrative and fulfilling career choice.
If you're just beginning your journey, consider pursuing relevant certifications, specializing in a therapeutic area, and building up your experience through internships to enhance your salary prospects. As you grow in this role, so will your earning potential, paving the way for an impactful and financially rewarding career in clinical research.
Reference Links:
National Institutes of Health (NIH) – Clinical Research Associate Career Path
ClinicalTrials.gov – A Service of the U.S. National Library of Medicine
Relevant Course Links:
Clinical Trial Management System
Clinical trials are a cornerstone of medical advancements, offering new insights into treatments, medications, and therapies. However, managing clinical trials is not without challenges. Trials often involve large volumes of data, complex regulatory requirements, multiple stakeholders, and stringent timelines. Delays, errors in data collection, and lapses in compliance can result in costly setbacks and even trial failure.
A Clinical Trial Management System (CTMS) is designed to address these challenges. By providing a centralized platform for planning, managing, and tracking clinical trials, a Clinical Trial Management System helps streamline processes, improve data accuracy, and ensure compliance with regulatory standards. In today's fast-paced medical research environment, adopting a CTMS is no longer an option but a necessity for successful trial management.
In this guide, we’ll explore what a Clinical Trial Management System is, its key features and benefits, the challenges of implementing one, and how advancements like AI and cloud technologies are paving the way for new advancements in managing clinical trials.
What Is a Clinical Trial Management System (CTMS)?
A Clinical Trial Management System (CTMS) is a software solution specifically designed to assist in the planning, tracking, and execution of clinical trials. CTMS platforms act as a centralized hub for all trial-related activities, providing tools for everything from participant recruitment and site management to budgeting and regulatory reporting.
The primary purpose of a Clinical Trial Management System is to streamline the complexities involved in clinical trial management by automating routine tasks and consolidating trial data into one place. For instance, managing trial timelines, collecting patient data, and ensuring regulatory compliance can be incredibly time-consuming when done manually. A CTMS reduces this workload, allowing research teams to focus more on conducting the trial rather than managing logistics.
A well-designed Clinical Trial Management System covers the entire lifecycle of a clinical trial, from planning to post-trial reporting. It integrates seamlessly with other trial management tools like Electronic Data Capture (EDC) systems and electronic Trial Master Files (eTMF), ensuring that data is captured accurately and stored securely.
Key Features of a Clinical Trial Management System
A comprehensive Clinical Trial Management System should include a wide range of features to effectively manage clinical trials. Let’s explore some of the most essential ones:
Trial Planning and Tracking: One of the key functions of a Clinical Trial Management System is to facilitate detailed planning and tracking of clinical trials. This includes timeline creation, milestone tracking, and overall project management. It allows for real-time tracking of key performance indicators (KPIs), ensuring that the trial remains on schedule.
Document Management: Clinical trials generate an overwhelming amount of documentation, from trial protocols to regulatory submissions. A CTMS offers a centralized location for managing, storing, and retrieving these documents. This is particularly beneficial in maintaining compliance, as missing or incorrect documentation can lead to regulatory penalties.
Participant Recruitment and Management: Recruiting participants is one of the most critical aspects of clinical trials. A Clinical Trial Management System helps manage the entire recruitment process, tracking participant eligibility, consent forms, and status throughout the trial. This feature reduces the risk of losing participants due to inefficient communication or data tracking.
Budget and Financial Management: Clinical trials are expensive, and managing the budget is crucial for their success. A Clinical Trial Management System offers tools for monitoring expenses, tracking trial-related costs, and managing payments to investigators and vendors. By providing detailed financial reports, a CTMS ensures that the trial stays within budget.
Regulatory Compliance and Reporting: Compliance with regulatory agencies such as the FDA, EMA, or local IRBs is mandatory for clinical trials. A CTMS helps manage compliance by providing audit trails, tracking submission deadlines, and generating necessary reports for regulators. This ensures that the trial remains compliant at all stages.
Communication Tools: Effective communication among stakeholders is crucial for trial success. A Clinical Trial Management System often includes built-in communication features such as task assignments, automated notifications, and shared dashboards to keep everyone on the same page.
Benefits of Using a Clinical Trial Management System
The benefits of implementing a Clinical Trial Management System go far beyond basic trial management. By automating routine tasks and centralizing trial data, a CTMS significantly enhances the efficiency of clinical trials. Here are some key benefits:
Improved Operational Efficiency: By automating manual processes, a Clinical Trial Management System helps streamline trial operations. Routine tasks such as scheduling, data entry, and reporting can be automated, freeing up valuable time for researchers and trial coordinators.
Enhanced Data Accuracy and Reduced Errors: Human errors in data entry can lead to significant consequences in clinical trials. A CTMS ensures data consistency and accuracy by automating data collection and reducing the need for manual input. This also improves the integrity of the trial data.
Real-Time Access to Data: A Clinical Trial Management System provides real-time access to critical trial data, allowing researchers and stakeholders to make informed decisions quickly. This feature is especially useful for identifying potential issues early and addressing them before they escalate.
Additional benefits :
Better Regulatory Compliance: A CTMS simplifies the process of meeting regulatory requirements by providing audit trails, compliance checklists, and pre-built templates for regulatory reports. This reduces the risk of non-compliance and helps avoid delays in trial approval.
Improved Collaboration: With a Clinical Trial Management System, all stakeholders, including sponsors, investigators, and regulatory bodies, have access to the same up-to-date information. This fosters better collaboration and communication across teams, ensuring that everyone is aligned on trial objectives and timelines.
Centralized Document and Data Management: Having all trial-related data and documents in one secure location simplifies trial management and ensures that all information is easily accessible. This reduces the time spent searching for documents and helps maintain compliance with regulatory requirements.
How to Choose the Right Clinical Trial Management System
Selecting the right Clinical Trial Management System for your organization requires careful consideration. Not all CTMS platforms offer the same features, and the needs of your clinical trial may vary depending on its complexity. Here are some key factors to consider:
Scalability: Ensure that the Clinical Trial Management System you choose can scale according to the size and scope of your trials. Whether you are conducting a small local study or a large multi-site global trial, the system should be flexible enough to grow with your needs.
Integration with Other Systems: A CTMS should seamlessly integrate with other key systems such as Electronic Data Capture (EDC) tools, eTMF, and financial systems. This ensures that data flows smoothly between different platforms, reducing the need for manual data entry and improving data accuracy.
User-Friendliness: The system should be easy to use and require minimal training. A complex system with a steep learning curve can lead to delays in adoption, which can slow down the trial process.
Cost and ROI: While the cost of implementing a Clinical Trial Management System can be significant, it’s essential to consider the long-term return on investment. A well-chosen CTMS can significantly reduce operational costs by automating routine tasks, improving trial efficiency, and preventing costly delays.
Challenges and Solutions in Implementing a Clinical Trial Management System
While implementing a Clinical Trial Management System can offer substantial benefits, the process is not without its challenges. Understanding these challenges and how to overcome them is crucial for a successful rollout:
Data Migration from Legacy Systems: One of the biggest challenges is migrating data from old systems to the new CTMS. To address this, develop a phased migration strategy that ensures data integrity and minimizes disruptions.
User Adoption: Getting staff to fully adopt the new system can be challenging, particularly if they are accustomed to using legacy systems. Offering comprehensive training and ongoing support is essential to ensure smooth adoption.
Customization: Not all clinical trials are alike. Ensure that your Clinical Trial Management System can be customized to fit the specific needs of your trials, including unique regulatory requirements or specialized workflows.
Future Trends in Clinical Trial Management Systems
As technology evolves, Clinical Trial Management Systems are also advancing. Here are some future trends to watch for:
AI and Machine Learning: Artificial Intelligence (AI) and machine learning are transforming clinical trial management by enabling predictive analytics and improving data-driven decision-making. These technologies can predict potential risks in trials, identify ideal participant populations, and optimize trial workflows.
Cloud-Based and SaaS Solutions: Cloud-based Clinical Trial Management Systems offer greater flexibility, allowing users to access the system from anywhere. SaaS (Software as a Service) models reduce the need for costly on-premise infrastructure, making CTMS solutions more accessible to smaller organizations.
Wearable Technology Integration: As wearable health devices become more common, Clinical Trial Management Systems are increasingly integrating with these technologies to collect real-time patient data. This not only improves data accuracy but also enhances patient engagement.
Conclusion
A Clinical Trial Management System (CTMS) is an invaluable tool for streamlining clinical trials, improving data management, and ensuring regulatory compliance. As clinical trials continue to grow in complexity, adopting a robust CTMS is essential for maintaining efficiency and reducing operational costs. With advancements in AI, cloud computing, and wearable technologies, the future of Clinical Trial Management Systems looks even more promising. By choosing the right CTMS for your organization, you can optimize your clinical trial processes and bring innovative treatments to market faster.
The Ultimate Guide to Becoming a Clinical Research Associate
Discover the steps, skills, and benefits of becoming a successful Clinical Research Associate. Explore career growth, certifications, and opportunities in clinical research.
A clinical research associate (CRA) plays a vital role in the development of new medical treatments. They ensure that clinical trials are conducted ethically and according to protocols, monitoring the progress from start to finish. CRAs are essential for ensuring data integrity and regulatory compliance.
The demand for CRAs is increasing due to the growth of the pharmaceutical industry and more clinical research studies. This field offers many opportunities for career growth with competitive salaries and benefits.
To become a successful clinical research associate, it's important to have a strong understanding of various aspects of the job like GCP monitoring of clinical trials and familiarity with EU clinical trials registry.
To gain this knowledge and expertise, consider enrolling in specialized online certification courses offered by CCRPS. They provide flexible learning options designed specifically for individuals aiming to excel in clinical research.
What Does a Clinical Research Associate (CRA) Do?
A Clinical Research Associate (CRA) plays an integral role in the clinical research process, ensuring that clinical trials are conducted ethically, safely, and efficiently. CRAs are responsible for monitoring clinical trials and ensuring compliance with regulatory requirements and study protocols.
Key Responsibilities of a CRA:
Study Design and Protocol Development
CRAs assist in the development of study protocols, which outline the objectives, design, methodology, statistical considerations, and organization of a clinical trial. This ensures that the study is scientifically sound and regulatory compliant.
Site Selection and Initiation
They identify suitable sites for conducting clinical trials, assessing their qualifications and infrastructure. Once selected, CRAs initiate the sites by training site staff on study protocols and procedures.
Monitoring Visits
Regular site visits to monitor the progress of clinical trials are a key responsibility. During these visits, CRAs ensure data integrity by verifying source documents against case report forms (CRFs) and checking for protocol adherence.
Regulatory Compliance
Ensuring that all activities related to the trial comply with local and international regulations is critical. CRAs keep up-to-date with guidelines such as Good Clinical Practice (GCP) to maintain high standards throughout the research process.
Data Management
CRAs oversee data collection processes to ensure accuracy and completeness. They resolve any data discrepancies promptly to maintain data quality.
Safety Reporting
Monitoring participant safety is paramount. CRAs ensure that adverse events (AEs) and serious adverse events (SAEs) are reported in accordance with regulatory requirements, following pharmacovigilance practices.
Communication
Acting as a liaison between the sponsor, clinical sites, and regulatory bodies is another essential duty. Effective communication helps address issues swiftly and keeps all stakeholders informed about trial progress.
Documentation
Maintaining meticulous records of all aspects of the trial is crucial. CRAs document everything from visit reports to correspondence with investigative sites, ensuring transparency and traceability throughout the trial process.
These responsibilities highlight the importance of a CRA in maintaining the integrity of clinical trials. By managing these tasks effectively, CRAs contribute significantly to the advancement of medical knowledge and patient care.
Why Should You Consider a Career as a Clinical Research Associate?
High Demand for CRAs
The pharmaceutical industry is experiencing rapid growth, resulting in an increased number of clinical research studies. Consequently, there is a significant demand for skilled Clinical Research Associates (CRAs). These professionals play a vital role in overseeing clinical trials, ensuring adherence to regulatory guidelines and generating reliable data.
Opportunities for Career Advancement
A career as a Clinical Research Associate offers numerous opportunities for advancement. CRAs can transition into various management positions or specialize in specific research areas. Some potential career paths include:
Clinical Trial Manager: Overseeing multiple clinical trials and managing teams of CRAs.
Regulatory Affairs Specialist: Ensuring compliance with local and international regulations.
Project Manager: Coordinating all aspects of a clinical trial from start to finish.
These roles not only offer professional growth but also enable CRAs to make significant contributions to medical science.
Attractive Salary Packages and Benefits
CRAs receive competitive salaries and comprehensive benefits. Reports indicate that entry-level CRAs can earn attractive salaries which increase with experience and certifications.
Some benefits commonly provided to CRAs include:
Health Insurance: Comprehensive medical coverage.
Retirement Plans: Contributions towards retirement savings.
Paid Time Off: Generous leave policies.
The combination of financial rewards and benefits makes this career option appealing for individuals who seek fulfilling work while also achieving their personal financial goals.
For those interested in the field, it's worth exploring the resources available at CCRPS for insights on the latest trends, as well as the opportunities offered by contract research organizations in India.
The Path to Becoming a Successful Clinical Research Associate
1. Assessing Your Suitability for the Role
Before you embark on a career as a Clinical Research Associate (CRA), it's important to evaluate whether this path is right for you. Take some time to assess your interests, skills, and qualifications to determine if becoming a CRA aligns with your aspirations.
Exploring Your Interests
Passion for Science and Medicine: A strong interest in medical research and scientific inquiry is crucial. CRAs work closely with clinical trials that aim to improve healthcare outcomes.
Attention to Detail: The role involves meticulous documentation and adherence to protocols. If you enjoy tasks that require careful attention to detail, this could be a good fit.
Problem-Solving Skills: CRAs often face unexpected challenges during clinical trials. Having a knack for problem-solving can make the job more rewarding.
Evaluating Your Skills
Communication Skills: Effective communication with various stakeholders, including researchers, sponsors, and regulatory bodies, is vital.
Organizational Abilities: CRAs juggle multiple tasks at once, so strong organizational skills are necessary.
Technical Proficiency: Familiarity with data management systems and clinical trial software is beneficial.
Reviewing Your Qualifications
Educational Background: Most CRAs have degrees in life sciences or related fields. Consider if your educational background aligns with these requirements.
Experience in Clinical Research: Prior experience in clinical research roles, such as internships or volunteer work, can be advantageous.
For those seeking additional insights on gaining relevant experience, you may find it helpful to explore resources on how to get clinical trial experience here.
Additionally, for those interested in advancing their knowledge in specific areas, there are management forums that offer courses like the Management Forum Advanced Pharmacovigilance.
By conducting this thorough self-assessment, you can gain clarity on whether pursuing a career as a CRA aligns with your goals and strengths.
This foundational step sets the stage for acquiring the necessary education and skills needed to succeed in this field.
2. Acquiring the Necessary Education and Skills
Importance of an Academic Degree
Starting a career as a CRA requires a solid educational foundation. Obtaining an academic degree in a relevant field such as Life Sciences, Biology, or Health Sciences is essential.
This not only demonstrates your commitment to the field but also equips you with the fundamental knowledge needed to understand complex clinical research processes.
Medical Terminology Knowledge
A strong grasp of medical terminology is crucial for anyone aspiring to become a CRA. Clinical research involves intricate medical details that require precise understanding and communication. Developing this knowledge through coursework or specialized training programs is beneficial.
Courses offered by CCRPS provide comprehensive training in medical terminology specific to clinical research, which can greatly enhance your medical terminology proficiency.
Understanding Regulations
Clinical trials are governed by strict local and international regulations. Familiarizing yourself with these regulations is critical for ensuring compliance and maintaining the integrity of research data.
Training programs often include modules on regulatory affairs, helping you stay updated on guidelines such as ICH GCP (International Council for Harmonisation Good Clinical Practice).
Education and skill development are foundational steps in becoming a successful CRA. By focusing on obtaining relevant academic qualifications, mastering medical terminology, and understanding regulatory requirements, you set yourself up for a rewarding career in clinical research.
3. Gaining Experience in the Field of Clinical Research
Gaining practical experience is crucial for anyone looking to build a career as a Clinical Research Associate (CRA). This hands-on exposure not only enhances your resume but also provides you with invaluable insights into the daily responsibilities and challenges of the role.
Internships, Volunteer Work, and Certificate Courses
Taking up internships or volunteer positions in research organizations or healthcare facilities can offer a solid foundation. These opportunities allow you to:
Observe real-world clinical trials
Interact with experienced professionals
Understand regulatory requirements
Certificate courses in areas such as pharmacovigilance and pharmacoepidemiology offered by reputable institutions like CCRP can also significantly enhance your knowledge and make you more competitive in the field.
Volunteering can demonstrate your commitment to the field, making you a more attractive candidate for future employers.
Entry-Level Positions
Starting in entry-level roles such as Clinical Trial Assistant or Data Coordinator is another effective strategy. These positions often involve:
Monitoring data quality
Assisting in patient recruitment
Supporting CRAs in their tasks
Such roles help you build relevant experience while gaining an understanding of the intricacies involved in clinical research.
Personal Assessment
Before diving into these experiences, conducting a personal assessment can be beneficial. Ask yourself:
Do I have strong organizational skills?
Am I detail-oriented?
Can I handle multiple tasks efficiently?
This self-assessment helps ensure that a career as a CRA aligns with your personal strengths and career aspirations.
Investing time in gaining practical experience through internships, volunteer work, or entry-level positions not only strengthens your resume but also prepares you for the complexities of a CRA role.
Benefits of Becoming Certified
Getting certified as a CRA comes with several advantages:
Increased Job Prospects: Employers often prefer candidates who are certified, as it assures them of the applicant's competence and dedication to the field. This can make your job applications more competitive.
Professional Credibility: Certification provides an official acknowledgement of your skills and knowledge, enhancing your credibility among peers and employers.
Career Advancement: With certification, you position yourself for advanced roles within clinical research, opening doors to management positions or specialized areas of interest.
Investing in certification is a strategic move for those serious about a career as a CRA. It not only boosts your qualifications but also instills confidence in potential employers regarding your capability to manage complex clinical trials effectively.
For those looking to deepen their understanding of medical efficacy definitions or find clinical trials for specific conditions like cancer, additional resources can be found through CCRPS, an organization that offers valuable insights and expertise in the field.
They also provide specific resources like a comprehensive medical efficacy definition and guidance on how to find clinical trials for cancer.
Certifications are more than just credentials; they are gateways to numerous opportunities and professional growth in the dynamic world of clinical research.
5. Nailing the CRA Job Application Process
Finding and applying for CRA positions requires strategic planning and preparation. Here are some essential steps to enhance your job application process:
Strategies for Finding and Applying for CRA Positions
Leverage Online Job Portals:
Websites like Indeed, Glassdoor, and LinkedIn are great places to start.
Set up job alerts to receive notifications about new postings.
Networking:
Join professional groups on LinkedIn and attend industry conferences.
Connect with professionals in the field through platforms like CCRPS's alumni network.
Creating a Strong Resume and Cover Letter:
Resume Tips:
Highlight relevant education, certifications, and experience.
Use keywords from the job description to pass Applicant Tracking Systems (ATS).
Quantify achievements where possible (e.g., "Monitored over 50 clinical trials with a 98% compliance rate").
Cover Letter Tips:
Tailor each cover letter to the specific job and company.
Demonstrate your understanding of the role and how your skills align.
Mention any relevant training or certifications from CCRPS.
Preparing for Interviews with Potential Employers
Research the Company:
Understand their mission, values, and recent projects.
Familiarize yourself with their clinical trial focuses, such as cancer drug trials.
Showcase Your Knowledge:
Be prepared to discuss clinical trial phases, Good Clinical Practice (GCP), and regulatory requirements.
Share examples of how you've applied your knowledge in practical settings.
Behavioral Interview Questions:
Practice responses to questions about teamwork, problem-solving, and conflict resolution.
Use the STAR method (Situation, Task, Action, Result) to structure your answers.
Technical Skills Demonstration:
You might be asked about specific tools or software used in clinical research.
Mention any hands-on experience you have with Electronic Data Capture (EDC) systems or other relevant technologies.
By following these strategies, you'll position yourself as a strong candidate in your career as a CRA.
6. Continuing Your Professional Development as a CRA
Staying updated on industry advancements is vital for a successful career as a Clinical Research Associate (CRA). Engaging in continuous professional development ensures that you remain knowledgeable about the latest trends, regulations, and best practices in clinical research.
Participation in Workshops and Conferences
Workshops: Attending workshops allows CRAs to gain hands-on experience and practical skills. These sessions often cover new methodologies, regulatory updates, and emerging technologies in clinical trials.
Conferences: Industry conferences provide valuable networking opportunities. They bring together professionals from various sectors of clinical research, fostering knowledge exchange and professional growth.
Ongoing Training Opportunities
Online Courses: Enrolling in online courses can be a flexible way to stay current with industry standards. CCRPS offers a range of specialized certification courses like CRA, CRC, and ICH GCP that are designed to enhance your expertise.
In-House Training Programs: Many organizations offer internal training programs tailored to their specific needs. Participating in these programs can help CRAs stay aligned with their employer's expectations and protocols.
Self-Assessment and Personal Growth
Regular self-assessment helps CRAs identify areas for improvement and set career goals. Exploring personal interests and strengths can guide you towards specialized areas within clinical research, such as data management or regulatory affairs.
For more insights on maintaining industry relevance, consider exploring resources like our clinical trial monitoring plan SOPs or understanding salary trends through our clinical trial assistant salary guide.
These guides can offer additional context for your ongoing professional development.
Embracing continuous learning not only enhances your competency but also boosts your career prospects. As the clinical research landscape evolves, being proactive about professional growth ensures you remain a valuable asset to any research team.
How CCRPS Can Help You Become a Clinical Research Associate Faster
Comprehensive Online Certification Courses
CCRPS offers specialized online certification courses tailored to the needs of aspiring Clinical Research Associates (CRAs). These courses include:
ICH Good Clinical Practice (GCP) Certification
Benefits of Choosing Online Learning
Opting for online certification through CCRPS brings multiple advantages:
Flexibility: Learn at your own pace, fitting coursework around your existing schedule. This is particularly beneficial for those balancing current jobs or academic commitments.
Accessibility: Access course materials from anywhere in the world. No need to relocate or commute, making it easier to integrate learning into your daily life.
Online learning also allows you to revisit course materials as needed, ensuring you fully grasp each topic before moving on.
Ensuring Industry Relevance Through Updated Curriculam
CCRPS ensures its courses reflect the latest industry standards and best practices. The curriculum is continually updated, incorporating new developments in clinical research techniques and regulatory guidelines.
This commitment to excellence ensures that graduates are well-prepared for the evolving demands of the field.
Graduates from CCRPS often share success stories, highlighting how these courses have facilitated their transition into CRA roles.
For instance, many have noted that the comprehensive nature of CCRPS training gave them a competitive edge in job applications and interviews.
Recognition and Trust in the Field
CCRPS is widely recognized as a credible training provider within the clinical research community. Many leading research organizations and agencies prefer candidates who have completed certification programs from CCRPS.
This recognition not only enhances your resume but also increases your professional credibility.
Choosing CCRPS for your certification means aligning yourself with a trusted institution known for producing competent and knowledgeable CRAs.
The industry trust in CCRPS graduates is a testament to the quality and relevance of their training programs.
By selecting CCRPS for your CRA certification, you are making a strategic decision to boost your career prospects and prepare for a successful future in clinical research.
Conclusion
Choosing a career as a Clinical Research Associate (CRA) opens doors to many opportunities for growth, competitive salaries, and the satisfaction of contributing to medical advancements. The path to becoming a CRA may seem challenging, but with hard work and the right guidance, you can achieve your goals.
CCRPS is here to support you throughout this journey. We offer comprehensive online certification courses designed specifically for clinical research roles, providing you with:
Flexibility: Learn at your own pace through our accessible online modules.
Up-to-date Knowledge: Stay informed about the latest practices and standards in clinical research.
Professional Recognition: Earn certifications that are widely acknowledged by top research organizations and agencies.
With CCRPS's resources, you can turn your dreams into reality. Join thousands of successful graduates who have advanced their careers in clinical research through our programs.
Contact us today and become a part of the dynamic field of clinical research associates.
FAQs (Frequently Asked Questions)
What is the role of a Clinical Research Associate (CRA)?
A Clinical Research Associate (CRA) is responsible for overseeing and monitoring clinical trials conducted by pharmaceutical companies, contract research organizations (CROs), or academic medical centers. Their role involves ensuring that the trials are conducted in compliance with regulatory standards, protocols, and good clinical practice (GCP). CRAs also verify the accuracy of data collected during the trials and communicate with the study site staff to address any issues that may arise.
Why should I consider a career as a Clinical Research Associate?
Becoming a Clinical Research Associate offers numerous opportunities for career growth and development. The pharmaceutical industry's continuous expansion and the rise in clinical research studies have created a high demand for CRAs. This demand translates into attractive salary packages, benefits, and opportunities for advancement into management roles or specialized areas of research.
How can I become a successful Clinical Research Associate?
Becoming a successful Clinical Research Associate involves several key steps. These include assessing your suitability for the role by exploring your interests, skills, and qualifications; acquiring the necessary education and skills in fields such as Life Sciences and medical terminology; gaining relevant experience through internships or entry-level positions; considering certification programs offered by reputable organizations CCRPS nailing the job application process through effective resume crafting and interview preparation; and continuing your professional development through ongoing training opportunities.
What does CCRPS offer to help me become a Clinical Research Associate?
CCRPS provides comprehensive online certification courses tailored to individuals aspiring to become Clinical Research Associates. These courses cover essential knowledge areas such as CRA, CRC, and ICH GCP. By choosing online learning with CCRPS, you can benefit from flexibility and accessibility while gaining industry-relevant skills. Additionally, CCRPS ensures that their curricula reflect the latest industry standards and best practices, thereby accelerating your journey towards becoming a successful CRA.
How does CCRPS ensure industry relevance through its courses?
CCRPS maintains industry relevance by enhancing its course curricula to reflect the latest standards and best practices in clinical research. Graduates of CCRPS courses have successfully transitioned into CRA roles, showcasing the effectiveness of CCRPS training in preparing individuals for careers in clinical research.
Why should I trust CCRPS as a training provider for Clinical Research Associate certification?
CCRPS is recognized as a trusted training provider by leading research organizations and agencies within the field of clinical research. The credibility of CCRPS is evidenced by its success stories of graduates who have excelled in their careers as CRAs after completing the specialized online certification courses offered by CCRPS.
Do You Know What Clinical Trials and Studies Can Offer?
Learn about the benefits, phases, and importance of clinical trials and studies in advancing medical research and improving patient care.
Clinical trials and clinical studies are essential parts of medical research. These investigations aim to answer specific questions about the safety, effectiveness, and best use of medications, medical devices, or treatments.
Definition and Significance of Clinical Trials and Studies
Clinical Trials: These are research studies conducted on human participants to evaluate the effects of new medical interventions. The main goal is to determine whether a new treatment is safe and effective.
Clinical Studies: This broader term includes clinical trials but also covers observational studies where researchers observe outcomes without intervening with treatments.
These studies are crucial for advancing medical knowledge and improving patient care. They help identify better ways to prevent, diagnose, and treat diseases.
Brief Overview of the Article
This article will explore various aspects of clinical trials and studies:
Understanding the distinction between observational studies and clinical trials
Discussing different phases of clinical trials
Highlighting the importance of participant involvement
Emphasizing the need for diversity in clinical trial populations
Promoting transparency through trial registries
Providing a comprehensive look at ClinicalTrials.gov as a valuable resource
CCRPS is dedicated to providing professional training for those interested in contributing to these vital research areas.
Whether you're a career changer or looking to advance in roles such as clinical research associate or coordinator, understanding clinical trials can be highly beneficial.
Understanding Clinical Trials
Explaining the Distinction Between Observational Studies and Clinical Trials
When discussing types of clinical studies, it's crucial to distinguish between observational studies and clinical trials. Both play vital roles in medical research, but they serve different purposes and follow distinct methodologies.
Observational Studies: These studies involve monitoring participants without intervening or altering their behavior. Researchers observe outcomes in a natural setting to understand correlations and potential causes of health-related events. For example, an observational study might investigate the link between diet and heart disease by tracking participants’ eating habits over several years.
Clinical Trials: Unlike observational studies, clinical trials are interventional. They test new treatments, drugs, or procedures by assigning participants to different interventions and comparing the outcomes. This controlled environment helps determine the efficacy and safety of new medical advancements.
Comprehensive Discussion on Different Phases of Clinical Trials
Clinical trials are conducted in phases, each designed to answer specific research questions while ensuring participant safety. Here’s a breakdown of what each phase entails:
Phase 1: Introduction and Key Objectives
Objective: Assess the safety, dosage, and side effects of a new drug or treatment.
Participants: Typically involves 20-100 healthy volunteers or patients.
Focus: Determining the highest dose that can be administered safely without severe side effects.
Outcome: Identification of safe dosage ranges and initial understanding of how the drug interacts with the human body.
For instance, a Phase 1 trial for a new cancer drug would focus on determining how much of the drug patients can take without serious harm.
Phase 2: Introduction and Key Objectives
Objective: Evaluate the efficacy and further assess safety.
Participants: Involves a larger group of 100-300 patients who have the condition the drug is meant to treat.
Focus: Assessing how well the drug works at treating the condition while continuing to monitor side effects.
Outcome: Preliminary data on effectiveness and short-term side effects; helps refine dosing regimens.
An example might be testing whether a new medication lowers blood pressure more effectively than existing treatments in patients with hypertension.
Phase 3: Introduction and Key Objectives
Objective: Confirm effectiveness, monitor side effects, compare with standard treatments, and collect information for safe usage.
Participants: Enrolls 300-3,000 participants across multiple locations.
Focus: Providing comprehensive evidence on effectiveness and identifying any adverse reactions compared to current standard treatments.
Outcome: Data used for regulatory approval from entities like the FDA or EMA.
To illustrate, Phase 3 trials might compare a new diabetes medication against a placebo or standard treatment across various demographics to ensure its broad applicability.
Phase 4: Introduction and Key Objectives
Objective: Gather additional information after approval to understand long-term effects.
Participants: Thousands of patients using the treatment in real-world settings.
Focus: Monitoring long-term safety, effectiveness, and any rare or unexpected side effects that may occur over time.
Outcome: Ensures continuous assessment of risk versus benefit in broader populations post-market approval.
A relevant example could be ongoing surveillance of a newly approved vaccine to track its performance across different age groups over several years.
These phases collectively ensure that any new treatment brought to market is backed by rigorous scientific evidence supporting its safety and efficacy. The commitment to thorough testing underscores why clinical trials are integral to advancing medical knowledge and improving patient care.
Importance of Participant Involvement
Understanding the motivations behind why individuals opt to participate in clinical trials can provide valuable insights into enhancing recruitment strategies. Participants often volunteer for several reasons:
1. Access to Cutting-edge Treatments
Many patients, particularly those with conditions that have limited treatment options, see participation as a way to access new and potentially life-saving therapies before they become widely available.
2. Contribution to Science and Medicine
A strong altruistic desire drives many participants. They wish to contribute to medical research that could help others in the future, even if the direct benefits to themselves are uncertain.
3. Close Monitoring and Care
Clinical trial participants often receive more frequent health check-ups and monitoring compared to standard care. This can be reassuring for those who want more comprehensive management of their condition.
4. Financial Compensation
Some trials offer financial incentives, which can be a motivating factor, especially for those in need of additional income.
Understanding the motivations behind why individuals opt to participate in clinical trials can provide valuable insights into enhancing recruitment strategies.
Participating in clinical trials is not without its benefits and risks. A clear understanding of both aspects can help potential volunteers make informed decisions.
Examining the Benefits and Risks for Participants in Clinical Trials
Benefits of Participating in Clinical Trials
Access to New Treatments: As mentioned earlier, participants may gain early access to treatments that are not yet available on the market.
Enhanced Medical Attention: The rigorous protocols of clinical trials necessitate close monitoring by healthcare professionals, ensuring participants receive high-quality care.
Contribution to Medical Advancements: Participants play a crucial role in advancing medical knowledge and improving future healthcare outcomes.
Potential Health Improvements: There is always a possibility that the new treatment being tested will be effective, providing health improvements for the participant.
Risks of Participating in Clinical Trials
Side Effects: New treatments may have unknown side effects that could be harmful or uncomfortable. Participants are monitored closely, but there is always an element of risk involved.
Placebo Effect: In some trials, participants might receive a placebo instead of the active treatment. This means they could miss out on potential benefits while still facing any associated risks.
Time Commitment and Convenience: Clinical trials often require frequent visits to the study site, which can be time-consuming and inconvenient for participants.
Emotional Impact: Dealing with uncertainties about treatment efficacy or potential side effects can be emotionally taxing.
Ensuring thorough eligibility screening helps minimize risks by confirming that only suitable candidates are selected for participation.
This screening process evaluates various factors such as medical history, current health status, and specific criteria related to the trial's objectives.
A well-informed participant involvement strategy considers these factors to improve both recruitment and retention rates in clinical trials.
Ensuring Diversity in Clinical Trials for Better Outcomes
Understanding the Need for Diverse Representation in Clinical Trial Populations
Clinical trials are essential for medical research as they provide crucial information about the effectiveness and safety of new treatments. It is important to have a diverse group of participants in these trials to ensure that the findings can be applied to a wide range of people. Without diversity, the results may be biased and could lead to treatments that are not as effective or even harmful for certain groups.
Here are some reasons why diverse representation is necessary in clinical trials:
Genetic Variability: Different genetic backgrounds can affect how individuals respond to medications. For example, some ethnic groups may process drugs differently due to genetic differences.
Disease Prevalence: Certain diseases may be more common in specific populations. By including these groups in clinical trials, we can ensure that the treatments are effective for everyone.
Socioeconomic Factors: Access to healthcare and lifestyle choices can vary among different socioeconomic groups. These factors can also influence how well a treatment works.
Challenges and Importance of Including Older Adults in Clinical Research
Older adults often have multiple health conditions and take various medications, making them a unique group in clinical research. Despite their significant healthcare needs, they are frequently not well-represented in trials.
Challenges of including older adults:
Health Complications: Older adults often have other health issues that can make it more difficult for them to participate in clinical trials.
Mobility Issues: Physical limitations can make it challenging for older adults to travel to the trial site for frequent visits.
Cognitive Decline: Conditions like dementia can affect their ability to understand and provide consent for participation.
Importance of including older adults:
Generalizable Results: Including older adults ensures that the findings from the trial are relevant to this age group, which is important considering their high use of healthcare services.
Safety Profiles: Older adults may process drugs differently in their bodies, so it is important to have specific safety information for this population.
Dosage Adjustments: Age-related changes in the body may require different dosage recommendations for older adults.
By including a diverse range of participants in clinical trials, we not only get more comprehensive data but also uphold principles of fairness by providing equal opportunities for all groups to benefit from new treatments. The CCRPS (Center for Clinical Research and Public Health Solutions) recognizes the significance of diversity in clinical research and offers extensive training programs to equip professionals with the skills needed to conduct inclusive and impactful studies.
"Inclusion of diverse populations in clinical trials is essential to ensure that medical interventions are safe and effective for everyone." - CCRPS
Next, we will explore how trial registries can improve transparency and accessibility in clinical trials.
Promoting Transparency and Accessibility through Trial Registries
Transparent and accessible clinical trial data are crucial for advancing medical research. The International Clinical Trials Registry Platform (ICTRP) plays a pivotal role in this domain. Managed by the World Health Organization (WHO), ICTRP aims to ensure that a complete view of research is accessible to all involved in healthcare decision-making.
Role of ICTRP in Facilitating Trial Registration and Data Transparency
1. Global Registry Network
ICTRP integrates data from multiple primary registries worldwide, including ClinicalTrials.gov, ensuring comprehensive coverage of clinical trials.
2. Standardization
By setting international standards for trial registration, ICTRP promotes consistency in the information reported across different registries.
3. Accessibility
ICTRP offers a unified search portal allowing researchers, healthcare professionals, and the public to access trial data easily. This platform supports informed decisions by providing detailed insights into ongoing and completed studies.
4. Ethical Compliance
Ensuring all trials are registered prevents selective reporting and publication bias, enhancing the reliability and credibility of research findings.
Key Features of ICTRP
Unified Search Portal: Users can search for trials across multiple registries using one platform.
Comprehensive Data: Information includes trial phase, participant demographics, interventions used, and outcomes measured.
Public Access: Open access to trial information ensures transparency and fosters trust among stakeholders.
Enhancing Research Quality
ICTRP's efforts address critical challenges in clinical research:
Avoiding Duplication: By providing a global overview of trials, ICTRP helps researchers avoid redundant studies.
Promoting Collaboration: Open data facilitates collaboration between researchers from different regions and disciplines, promoting innovation.
Informed Decisions: Healthcare providers can make better treatment decisions based on comprehensive data from registered trials.
The commitment to transparency and accessibility through platforms like ICTRP is fundamental to advancing global health research.
Conclusion
Understanding clinical trials and studies opens up a world of possibilities for advancements in medicine and healthcare. These trials are crucial for developing new treatments, understanding diseases, and improving patient care. By participating, eligible individuals can contribute to this vital research while potentially gaining access to new therapies.
Key Takeaways:
Clinical trials and studies are essential for medical progress.
Participation can offer personal health benefits and contribute to scientific knowledge.
Staying informed about ongoing clinical trials helps individuals find opportunities suited to their needs.
CCRPS provides comprehensive training programs for those looking to enter or advance in the field of clinical research.
With over 22,000 researchers trained, our courses are designed to equip you with the knowledge needed to excel in various roles within this critical industry.
In addition, if you want to be more qualified in the field of clinical research, you may want to consider getting certified in several key areas. A great starting point is the CCRPS’ online pharmacovigilance course, which helps new professionals improve their qualifications and gain expert insight in the field. This course is curated by real clinical research professionals and is flexible to your schedule.
If you’re aiming to further enhance your career, CCRPS offers a variety of specialized courses tailored to different roles within clinical research. Those interested in coordinating clinical trials might consider the Clinical Research Coordinator course. For those looking to oversee clinical trial conduct, the CRA (Clinical Research Associate) course is ideal.
Aspiring clinical trials assistants who support clinical research sites can benefit from the Clinical Trials Assistant Training. If you are pursuing leadership roles in clinical research projects, the Advanced Clinical Research Project Manager Certification might be the right choice.
For physicians who wish to lead clinical studies, the Advanced Principal Investigator Physician Certification can provide the necessary expertise. Furthermore, individuals interested in ensuring the safety and efficacy of clinical trials may find the Medical Monitor Certification extremely useful.
Finally, to stay compliant with international standards in clinical research, consider our ICH-GCP (International Conference on Harmonisation - Good Clinical Practice) course, which is crucial for anyone involved in global clinical research.
FAQs (Frequently Asked Questions)
What is the significance of clinical trials and studies?
Clinical trials and studies play a crucial role in testing the safety and effectiveness of new treatments, interventions, or medical devices before they are made available to the public. They help advance medical knowledge and improve patient care by providing evidence-based data on the benefits and risks of various healthcare approaches.
What are the different types of clinical studies?
Clinical studies can be categorized into observational studies and clinical trials. Observational studies observe participants in their natural environment without any intervention, while clinical trials involve testing a specific intervention or treatment on participants to evaluate its effects.
Why is participant involvement important in clinical trials?
Participant involvement is crucial in clinical trials as it allows researchers to gather real-world data on the safety and efficacy of new treatments. By volunteering for clinical trials, individuals contribute to the advancement of medical science and have the opportunity to access potentially life-saving therapies before they are widely available.
How does diversity in clinical trials contribute to better outcomes?
Diverse representation in clinical trial populations ensures that research findings are applicable to a broader range of individuals, leading to more generalizable results. Additionally, including older adults in clinical research is essential for understanding how treatments affect this demographic, ultimately improving healthcare for this population.
What is the role of International Clinical Trials Registry Platform (ICTRP) in facilitating trial registration and data transparency?
The ICTRP plays a vital role in promoting transparency and accessibility through trial registries by providing a platform for registering clinical trials from around the world. It enhances data transparency by making information on ongoing and completed trials easily accessible to the public, researchers, and healthcare professionals.
The Role and Importance of Clinical Research Coordinators
Clinical research coordinators (CRCs) are crucial in the healthcare and pharmaceutical industries. They manage clinical trials, ensuring they adhere to regulatory requirements and scientific standards. Their role involves coordinating between different stakeholders, including patients, healthcare providers, and researchers. This blog delves into the responsibilities, qualifications, and career prospects of CRCs, with a particular focus on their salaries, especially at renowned institutions like Massachusetts General Hospital.
Responsibilities of Clinical Research Coordinators
Clinical research coordinators perform various tasks to ensure clinical trials' smooth and efficient conduct. Their responsibilities include:
Study Planning and Coordination: CRCs assist in developing study protocols, managing study timelines, and coordinating various trial phases.
Regulatory Compliance: They ensure all trials comply with regulatory requirements, including obtaining necessary approvals from Institutional Review Boards (IRBs).
Data Management: CRCs are responsible for data collection, entry, and maintenance, ensuring data integrity and confidentiality.
Patient Recruitment and Communication: They recruit and screen potential study participants, provide informed consent, and maintain ongoing communication with participants throughout the study.
References
Qualifications and Skills Required
To become a clinical research coordinator, certain educational and professional qualifications are typically required:
Educational Background: A bachelor's degree in life sciences, nursing, or a related field is usually required. Advanced degrees or certifications can be advantageous.
Certifications: Professional certifications such as the Certified Clinical Research Coordinator (CCRC) can enhance job prospects and credibility.
Skills: Essential skills include strong organizational abilities, attention to detail, excellent communication skills, and proficiency in using clinical trial management software (CTMS).
References
Career Prospects and Salary
The career prospects for clinical research coordinators are promising, with a growing demand for clinical trials. Salaries can vary widely based on experience, education, and location. For example, clinical research coordinators at Massachusetts General Hospital enjoy competitive salaries due to the institution's prestigious reputation.
Salary Insights at Massachusetts General Hospital
At Massachusetts General Hospital, clinical research coordinators can expect to earn salaries that reflect their expertise and the institution's standards. According to recent data, the average salary for a CRC at Massachusetts General Hospital is around $60,000 per year, with opportunities for growth based on experience and additional qualifications.
References
The Impact of Clinical Research Coordinators
Clinical research coordinators play a pivotal role in advancing medical research and improving patient care. Their contributions ensure the successful implementation of clinical trials, leading to the development of new treatments and therapies.
Key Contributions
Advancing Medical Knowledge: By managing clinical trials, CRCs contribute to the generation of new medical knowledge and innovations.
Ensuring Patient Safety: They ensure that trials are conducted safely and ethically, prioritizing patient welfare.
Facilitating Regulatory Approval: CRCs help navigate the complex regulatory landscape, facilitating the approval of new drugs and treatments.
References
Challenges Faced by Clinical Research Coordinators
Despite the rewarding nature of their work, clinical research coordinators face several challenges:
Regulatory Hurdles: Navigating complex regulatory requirements can be challenging and time-consuming.
Participant Recruitment: Recruiting and retaining study participants can be difficult, impacting study timelines.
Data Management: Ensuring the accuracy and confidentiality of clinical trial data is crucial and demanding.
References
Enhancing the Role of Clinical Research Coordinators
To address these challenges and enhance the role of CRCs, several strategies can be implemented:
Training and Education: Continuous training and education programs can help CRCs stay updated with regulatory changes and new methodologies.
Technology Integration: Utilizing advanced clinical trial management systems can streamline data management and improve efficiency.
Collaboration and Support: Encouraging collaboration among stakeholders and providing adequate support can alleviate some of the challenges CRCs face.
References
Future Trends in Clinical Research Coordination
The field of clinical research coordination is evolving, with several trends shaping its future:
Increased Use of Technology: Technology, including artificial intelligence and machine learning, is increasingly being integrated into clinical trials to enhance efficiency and accuracy.
Focus on Patient-Centered Trials: There is a growing emphasis on patient-centered approaches, ensuring that trials are designed with the patient experience in mind.
Global Collaboration: International collaboration is becoming more common, enabling more extensive and diverse clinical trials.
References
Conclusion
Clinical research coordinators are integral to the success of clinical trials, playing a vital role in advancing medical research and improving patient care. Their contributions, challenges, and future prospects highlight the importance of this profession. As the field continues to evolve, CRCs will remain at the forefront of medical innovation, ensuring the safe and effective conduct of clinical trials.
The Role of Physician Assistants in Clinical Research
Role of Physician Assistants in Clinical Research
The healthcare industry is a dynamic field that continuously evolves with new research and advancements. Physician assistants (PAs) play a vital role in this landscape, not only in clinical settings but also in research. This blog delves into the involvement of physician assistants in clinical research, shedding light on their contributions, responsibilities, and the impact of their work on medical advancements.
Understanding the Role of Physician Assistants
Physician assistants are licensed medical professionals who practice under the supervision of physicians. They are trained to perform a wide range of medical tasks, from diagnosing and treating illnesses to prescribing medications. The scope of their practice can vary depending on the state or country regulations, but they generally work in various healthcare settings, including hospitals, clinics, and private practices.
Key Responsibilities of Physician Assistants
Conducting patient examinations
Diagnosing and treating illnesses
Performing medical procedures
Prescribing medications
Educating patients about preventive care
Assisting in surgeries
Collaborating with healthcare teams
Physician Assistants in Clinical Research
Clinical research is a crucial aspect of the medical field, leading to new treatments, medications, and improved patient care practices. Physician assistants are increasingly involved in clinical research, contributing their medical expertise and patient care skills to advance medical knowledge.
Involvement in Research Studies
Physician assistants can participate in various types of clinical research studies, including:
Clinical Trials: PAs can help design, conduct, and monitor clinical trials, ensuring they adhere to ethical standards and regulatory requirements.
Observational Studies: In these studies, PAs collect and analyze data from patient observations to identify trends and outcomes.
Translational Research: PAs bridge the gap between laboratory research and clinical applications, helping translate scientific discoveries into practical treatments.
Responsibilities in Research Settings
The specific roles of PAs in clinical research can vary, but their responsibilities often include:
Patient Recruitment: Identifying and enrolling eligible patients for research studies.
Data Collection: Gathering accurate and comprehensive data from patient interactions, medical records, and laboratory results.
Patient Monitoring: Ensuring patient safety and adherence to study protocols throughout the research process.
Data Analysis: Assisting in the interpretation of research data to draw meaningful conclusions.
Ethical Compliance: Ensuring that research studies comply with ethical guidelines and regulatory standards.
Benefits of PA Involvement in Research
The involvement of physician assistants in clinical research offers several benefits:
Improved Patient Care: PAs bring a patient-centered approach to research, focusing on the well-being and safety of participants.
Enhanced Data Accuracy: Their clinical expertise ensures accurate data collection and interpretation.
Increased Efficiency: PAs can streamline research processes, reducing the time and resources needed to conduct studies.
Broader Perspectives: The diverse backgrounds of PAs contribute to a more comprehensive understanding of research outcomes.
Challenges and Opportunities
While physician assistants have much to offer in clinical research, they also face certain challenges. Understanding these challenges and opportunities can help optimize their involvement in research settings.
Challenges
Time Constraints: Balancing clinical duties with research responsibilities can be demanding.
Training Requirements: Additional training in research methodologies and regulatory compliance may be necessary.
Limited Recognition: The contributions of PAs in research may not always be fully recognized or appreciated.
Opportunities
Professional Development: Engaging in research provides PAs with opportunities for professional growth and development.
Collaboration: Research settings offer a platform for PAs to collaborate with other healthcare professionals and researchers.
Impact on Healthcare: PAs can make significant contributions to medical advancements, improving patient outcomes and healthcare practices.
Case Studies and Examples
To illustrate the impact of physician assistants in clinical research, let's explore some real-world examples and case studies.
Case Study 1: PA-Led Clinical Trial
In a clinical trial investigating a new treatment for diabetes, a team of physician assistants played a crucial role. They were involved in patient recruitment, data collection, and patient monitoring. Their efforts ensured that the trial was conducted smoothly and ethically, leading to the successful development of a new medication that improved the lives of many patients.
Case Study 2: Observational Study on Hypertension
A group of PAs conducted an observational study to understand the long-term effects of hypertension in elderly patients. By collecting and analyzing patient data, they identified key factors contributing to improved blood pressure management. Their findings were published in a leading medical journal, influencing hypertension treatment guidelines.
Skills and Qualifications for Research-Involved PAs
Physician assistants who wish to engage in clinical research should possess specific skills and qualifications to excel in this field.
Essential Skills
Clinical Expertise: A strong foundation in clinical practice is essential for understanding research contexts and patient care.
Analytical Skills: Ability to analyze data, identify trends, and draw meaningful conclusions.
Attention to Detail: Precision in data collection and documentation to ensure accuracy and reliability.
Communication Skills: Effective communication with research teams, patients, and regulatory bodies.
Recommended Qualifications
Research Training: Additional training or certification in clinical research methodologies and regulatory compliance.
Advanced Degrees: Pursuing advanced degrees (e.g., Master's or PhD) in related fields can enhance research capabilities.
Experience: Gaining experience through participation in research projects or clinical trials.
Professional Development Opportunities
Physician assistants interested in clinical research can pursue various professional development opportunities:
Workshops and Seminars: Attending workshops and seminars on clinical research topics.
Certifications: Obtaining certifications from recognized institutions (e.g., Clinical Research Certification from the Association of Clinical Research Professionals).
Networking: Joining professional organizations and networks focused on clinical research.
Future Trends and Developments
The role of physician assistants in clinical research is expected to grow as the healthcare industry continues to evolve. Emerging trends and developments will shape their involvement in research settings.
Technological Advancements
Digital Health Tools: The use of digital health tools and technologies will enhance data collection, analysis, and patient monitoring.
Telemedicine: Telemedicine will enable PAs to conduct research remotely, expanding their reach and impact.
Artificial Intelligence: AI and machine learning will assist PAs in data analysis and predictive modeling.
Collaborative Research
Interdisciplinary Teams: PAs will collaborate with interdisciplinary teams, including physicians, nurses, and researchers, to conduct comprehensive studies.
Global Research Networks: Participation in global research networks will provide PAs with access to diverse patient populations and resources.
Policy and Regulatory Changes
Expanded Roles: Policy changes may expand the roles of PAs in research, recognizing their contributions and providing more opportunities.
Ethical Standards: Ongoing updates to ethical standards and regulatory guidelines will ensure the integrity and quality of clinical research.
Finalization
Physician assistants play a crucial role in clinical research, contributing their clinical expertise, patient care skills, and dedication to advancing medical knowledge. Their involvement in research offers numerous benefits, including improved patient care, accurate data collection, and efficient research processes. As the healthcare industry continues to evolve, the role of PAs in clinical research will become increasingly significant, shaping the future of medical advancements and patient outcomes.
References
American Academy of Physician Assistants. (2023). The Role of PAs in Clinical Research.
Clinical Research Society. (2022). Physician Assistants and Clinical Trials.
Journal of Clinical Research. (2021). Observational Studies Conducted by PAs.
National Institutes of Health. (2023). PA Contributions to Translational Research.
Association of Clinical Research Professionals. (2022). Certification Programs for PAs in Research.
Health Research Policy. (2022). Impact of PA-Led Research Studies.
Digital Health Journal. (2023). Technological Advancements in Clinical Research.
Global Research Network. (2021). Collaborative Research Opportunities for PAs.
Clinical Research Ethics. (2023). Ethical Standards in Clinical Research.
Medical Research Review. (2022). Future Trends in Clinical Research.
The Comprehensive Guide to Medical Research Consultant Jobs
Medical Research Consultant Jobs
Medical research consultants play a crucial role in the healthcare industry, bridging the gap between research and practical application. They provide expert advice, conduct studies, and help shape healthcare policies and practices. This blog explores the essential aspects of medical research consultant jobs, including the required skills, typical responsibilities, career paths, and the current job market trends.
Understanding the Role of a Medical Research Consultant
A medical research consultant is a professional who offers specialized knowledge and expertise in medical research to organizations such as pharmaceutical companies, hospitals, universities, and government agencies. These consultants are instrumental in designing, conducting, and analyzing clinical trials, ensuring that research findings are valid and applicable.
Key Responsibilities
Medical research consultants have a wide range of responsibilities, including:
Designing Research Studies: Developing research protocols and methodologies to ensure that studies are scientifically sound.
Conducting Research: Overseeing the implementation of research studies, including data collection and analysis.
Consulting with Clients: Providing expert advice to clients on research-related issues, including study design, regulatory compliance, and data interpretation.
Preparing Reports and Publications: Writing detailed reports and scientific papers based on research findings.
Regulatory Compliance: Ensuring that all research activities comply with relevant regulations and ethical standards.
Required Skills and Qualifications
To excel as a medical research consultant, one must possess a combination of educational qualifications, technical skills, and soft skills.
Educational Background: A minimum of a master's degree in a relevant field such as medical sciences, public health, or clinical research is typically required. A Ph.D. is often preferred.
Technical Skills: Proficiency in statistical analysis, research methodologies, and data interpretation. Familiarity with software such as SPSS, SAS, and R is advantageous.
Soft Skills: Strong communication, problem-solving, and project management skills. The ability to work collaboratively with diverse teams is essential.
Career Path and Advancement
The career path for a medical research consultant often begins with roles such as research assistant or clinical research coordinator. With experience, professionals can advance to senior consultant positions or take on leadership roles in research organizations.
Career Progression:
Entry-Level Positions: Research Assistant, Clinical Research Coordinator
Mid-Level Positions: Research Analyst, Clinical Research Associate
Senior-Level Positions: Senior Consultant, Principal Investigator, Research Director
The Job Market for Medical Research Consultants
The demand for medical research consultants is influenced by various factors, including advancements in medical technology, the increasing complexity of clinical trials, and the growing emphasis on evidence-based medicine.
Industry Demand
Several industries actively seek medical research consultants, including:
Pharmaceutical Companies: To develop and test new drugs.
Healthcare Providers: To improve patient care and treatment protocols.
Government Agencies: To shape public health policies and regulatory frameworks.
Academic Institutions: To conduct cutting-edge research and mentor students.
Job Outlook
The job outlook for medical research consultants is positive, with the Bureau of Labor Statistics projecting steady growth in related fields. This growth is driven by the continuous need for medical research to address emerging health challenges and improve healthcare outcomes.
Essential Tips for Aspiring Medical Research Consultants
For those aspiring to become medical research consultants, here are some essential tips to help you succeed in this competitive field:
Pursue Relevant Education and Training
Obtain a degree in medical sciences, public health, or a related field.
Consider advanced degrees or certifications, such as the Advanced Clinical Medical Scribe Certification Course.
Participate in internships and research projects to gain practical experience.
Build a Strong Professional Network
Join professional organizations, such as the Association of Clinical Research Professionals (ACRP) and the Society for Clinical Research Sites (SCRS).
Attend conferences, workshops, and seminars to stay updated on industry trends and connect with peers.
Develop Specialized Skills
Gain expertise in specific areas of medical research, such as oncology, cardiology, or infectious diseases.
Learn about regulatory requirements and ethical standards in medical research.
Stay Current with Industry Trends
Read industry journals, such as The Lancet and JAMA, to keep abreast of the latest research findings.
Follow thought leaders and experts on social media platforms like LinkedIn and Twitter.
Leverage Technology
Use advanced research software and tools to enhance your data analysis and reporting capabilities.
Familiarize yourself with electronic data capture (EDC) systems and clinical trial management systems (CTMS).
The Impact of Medical Research Consultants on Healthcare
Medical research consultants play a pivotal role in advancing healthcare by contributing to the development of new treatments, improving patient care, and shaping health policies. Their expertise is essential in translating research findings into practical applications that benefit society.
Contributions to Medical Advancements
Medical research consultants have been instrumental in several key advancements, including:
Drug Development: Assisting in the creation of new medications and therapies.
Clinical Guidelines: Helping to establish evidence-based guidelines for patient care.
Public Health Initiatives: Supporting initiatives to prevent and control diseases.
Enhancing Patient Outcomes
By ensuring that research studies are scientifically rigorous and ethically sound, medical research consultants help improve patient outcomes. Their work leads to the development of safer and more effective treatments, ultimately enhancing the quality of care.
Shaping Health Policies
Medical research consultants provide valuable insights that inform health policies and regulations. Their expertise helps ensure that policies are based on the latest scientific evidence and best practices, promoting public health and safety.
Challenges Faced by Medical Research Consultants
While the role of a medical research consultant is rewarding, it also comes with its challenges. Understanding these challenges can help aspiring consultants prepare for the demands of the job.
Navigating Regulatory Requirements
Medical research is heavily regulated, and consultants must navigate complex regulatory landscapes. Staying informed about the latest regulations and ensuring compliance can be challenging but is essential for conducting ethical and legal research.
Managing Ethical Considerations
Ethical considerations are paramount in medical research. Consultants must ensure that studies are conducted ethically, with the rights and well-being of participants protected. This requires a deep understanding of ethical principles and guidelines.
Balancing Multiple Projects
Medical research consultants often juggle multiple projects simultaneously. Effective time management and organizational skills are crucial to handle the workload and meet deadlines.
Adapting to Technological Advances
The rapid pace of technological advancements in medical research requires consultants to continuously update their skills and knowledge. Staying current with the latest tools and methodologies is vital for success in this field.
Section III: Key Takeaways for Aspiring Medical Research Consultants
For those looking to embark on a career as a medical research consultant, here are some key takeaways to keep in mind:
Educational and Professional Development
Invest in Education: Pursue relevant degrees and certifications to build a strong foundation in medical research.
Continuous Learning: Stay updated with the latest research, technologies, and industry trends.
Networking and Collaboration
Join Professional Organizations: Engage with industry groups to expand your network and access resources.
Collaborate with Experts: Work with experienced professionals to gain insights and mentorship.
Skills and Competencies
Develop Technical Skills: Gain proficiency in research methodologies, data analysis, and regulatory compliance.
Enhance Soft Skills: Improve communication, problem-solving, and project management abilities.
Industry Trends and Opportunities
Stay Informed: Follow industry news and developments to identify new opportunities and areas of growth.
Leverage Technology: Use advanced tools and software to streamline research processes and enhance productivity.
Conclusion
Medical research consultants play a vital role in advancing healthcare by bridging the gap between research and practice. They contribute to the development of new treatments, improve patient care, and shape health policies. Aspiring medical research consultants should focus on gaining relevant education, building a strong professional network, and continuously updating their skills to succeed in this dynamic and rewarding field.
References
Clinical Laboratory Practice Certification
Good Clinical Laboratory Practice (GCLP)
Good Clinical Laboratory Practice (GCLP) certification is an essential component in the realm of clinical research and laboratory management. It ensures that laboratories operate under strict quality standards, which guarantees the reliability and integrity of data produced. This certification not only enhances the credibility of a laboratory but also plays a critical role in protecting public health by ensuring the accuracy of clinical trials and research.
What is GCLP?
Good Clinical Laboratory Practice (GCLP) combines principles of Good Laboratory Practice (GLP) and Good Clinical Practice (GCP) to ensure that laboratories conducting clinical trials produce reliable, reproducible, and high-quality data. GCLP guidelines cover all aspects of laboratory operations, from sample collection and analysis to data recording and reporting.
Importance of GCLP Certification
GCLP certification is vital for several reasons:
Quality Assurance: Ensures that laboratory processes and data are consistent and reproducible.
Regulatory Compliance: Meets the regulatory requirements set by authorities such as the FDA and EMA.
Data Integrity: Maintains the accuracy and integrity of data used in clinical research.
Risk Management: Reduces the risk of errors and non-compliance, which can lead to costly delays or failures in clinical trials.
Enhanced Credibility and Trust
Obtaining GCLP certification significantly enhances a laboratory's credibility. It demonstrates a commitment to maintaining high standards and builds trust with sponsors, regulatory bodies, and the public.
Improved Laboratory Efficiency
Implementing GCLP guidelines streamlines laboratory processes, leading to increased efficiency and productivity. Standardized procedures reduce the likelihood of errors, saving time and resources in the long run.
Competitive Advantage
GCLP-certified laboratories are often preferred by sponsors and clients in the pharmaceutical and biotechnology industries. This certification can provide a competitive edge, attracting more business opportunities.
Safeguarding Public Health
By ensuring the accuracy and reliability of clinical data, GCLP certification plays a crucial role in safeguarding public health. Reliable data is essential for developing effective treatments and therapies.
Key Components of GCLP Certification
Personnel and Training
Ensuring that laboratory personnel are adequately trained is a cornerstone of GCLP certification. Continuous training programs keep staff updated on the latest techniques and regulatory requirements.
Standard Operating Procedures (SOPs)
SOPs are detailed, written instructions to achieve uniformity in the performance of a specific function. They are crucial for maintaining consistency and quality in laboratory processes.
Equipment Calibration and Maintenance
Regular calibration and maintenance of laboratory equipment are essential to ensure accurate and reliable results. GCLP guidelines require documented proof of equipment performance.
Documentation and Records
Accurate documentation is fundamental to GCLP compliance. This includes detailed records of all laboratory activities, from sample collection to data analysis and reporting.
Quality Control and Assurance
Implementing robust quality control measures ensures that laboratory results are consistent and reliable. Quality assurance processes verify that all aspects of laboratory operations meet the required standards.
Implementing GCLP in Your Laboratory
Initial Assessment
Conducting an initial assessment of current laboratory practices against GCLP standards is the first step towards implementation. This helps identify gaps and areas for improvement.
Training and Education
Investing in comprehensive training programs for laboratory staff is crucial. This ensures that everyone is aware of GCLP guidelines and understands their role in maintaining compliance.
Developing SOPs
Creating detailed SOPs for all laboratory processes is essential. These should be regularly reviewed and updated to reflect any changes in practices or regulations.
Regular Audits
Conducting regular internal and external audits helps ensure ongoing compliance with GCLP standards. Audits can identify potential issues before they become significant problems.
Continuous Improvement
GCLP implementation is an ongoing process. Continuous improvement initiatives, such as feedback loops and process reviews, help maintain high standards and adapt to new challenges.
The Impact of GCLP Certification on Clinical Research
Ensuring Data Integrity
One of the most significant impacts of GCLP certification is the assurance of data integrity. Reliable data is the foundation of clinical research, and GCLP ensures that data is accurate, consistent, and reproducible.
Enhancing Patient Safety
By maintaining high standards of laboratory practice, GCLP certification enhances patient safety. Accurate and reliable data is crucial for developing safe and effective treatments.
Facilitating Regulatory Approval
GCLP-certified laboratories are more likely to meet the stringent requirements of regulatory authorities. This can expedite the approval process for new treatments and therapies, bringing them to market faster.
Boosting Research Efficiency
Standardized procedures and robust quality control measures streamline laboratory operations, leading to increased efficiency and productivity in clinical research.
Laboratory Information Management Systems (LIMS)
Laboratory Information Management Systems (LIMS) play a vital role in achieving GCLP compliance. These systems automate data collection, management, and reporting, ensuring accuracy and traceability.
Automation and Robotics
The integration of automation and robotics in laboratory processes reduces the likelihood of human error and increases efficiency. Automated systems can handle repetitive tasks, freeing up staff for more complex activities.
Data Analytics and AI
Advanced data analytics and artificial intelligence (AI) tools can enhance data analysis and interpretation, leading to more accurate and insightful results. These technologies support GCLP compliance by ensuring data integrity and reliability.
Evolving Standards and Guidelines
As scientific research and technology continue to advance, GCLP standards and guidelines will evolve. Staying updated with these changes is essential for maintaining compliance and ensuring the highest quality of laboratory practice.
Global Harmonization
Efforts towards global harmonization of GCLP standards are underway. This will facilitate international collaboration and streamline the regulatory approval process for new treatments and therapies.
Emphasis on Data Security
With the increasing amount of data generated in clinical research, data security will become a more significant focus. GCLP guidelines will likely include more stringent requirements for protecting sensitive information.
Final Thought
Good Clinical Laboratory Practice (GCLP) certification is a cornerstone of quality and reliability in clinical research. By ensuring high standards of laboratory practice, GCLP certification enhances credibility, efficiency, and data integrity. Implementing GCLP guidelines is an ongoing process that requires continuous improvement and adaptation to new challenges. As technology and standards evolve, staying updated with GCLP requirements will be crucial for maintaining compliance and ensuring the highest quality of clinical research.
References
ICH GCP Guidelines - ICH Official Website
FDA Guidelines on GCLP - FDA Official Website
EMA GCP Compliance - EMA Official Website
WHO GCLP Guidelines - WHO Official Website
Laboratory Information Management Systems (LIMS) - LabWare
Quality Assurance in Clinical Laboratories - Journal of Clinical Laboratory Analysis
Clinical Research Fast Track Salar
Clinical Research Careers
The field of clinical research offers a rewarding career path for those interested in advancing medical knowledge and patient care. Clinical researchers play a pivotal role in the development of new medications, therapies, and medical devices. These professionals ensure that clinical trials are conducted safely, ethically, and effectively, making significant contributions to the health industry.
Why Choose a Career in Clinical Research?
Clinical research careers are not only fulfilling but also offer substantial financial rewards. As the demand for new treatments and medical advancements grows, so does the need for qualified clinical research professionals. This demand is reflected in the competitive salaries offered in this field.
Moreover, a career in clinical research provides:
Job Security: With the continuous development of new treatments, the need for clinical research professionals remains steady.
Professional Growth: Opportunities for advancement and specialization in various areas of clinical research.
Impactful Work: Contributing to the development of life-saving treatments and improving patient outcomes.
Understanding Clinical Research Fast Track Programs
Clinical research fast track programs are designed to accelerate the training process for aspiring clinical researchers. These programs provide intensive, focused training, allowing individuals to quickly gain the skills and knowledge necessary to enter the field.
Key Features of Fast Track Programs
Accelerated Learning: Fast track programs condense the curriculum into a shorter time frame without sacrificing the quality of education.
Comprehensive Training: These programs cover all essential aspects of clinical research, including regulatory requirements, clinical trial design, and data management.
Practical Experience: Many fast track programs include hands-on training and internships to provide real-world experience.
Benefits of Enrolling in a Fast Track Program
Quick Entry into the Workforce: Accelerated programs enable students to start their careers sooner.
High Earning Potential: Graduates of fast track programs can quickly begin earning competitive salaries.
Increased Job Opportunities: Comprehensive training and practical experience make graduates highly desirable to employers.
Clinical Research Fast Track Salary Overview
One of the primary attractions of a career in clinical research is the potential for high earnings. Salaries in this field can vary widely based on factors such as experience, education, and geographic location.
Factors Influencing Salary
Several key factors influence the salary of clinical research professionals:
Experience: More experienced researchers typically command higher salaries.
Education: Advanced degrees and specialized certifications can significantly boost earning potential.
Location: Salaries can vary depending on the cost of living and demand for clinical research professionals in different regions.
Employer Type: Positions in pharmaceutical companies, contract research organizations (CROs), and academic institutions may offer different salary scales.
Salary Ranges for Various Positions
The following table provides an overview of typical salary ranges for different positions within clinical research:
Growing Demand for Clinical Research Professionals
The clinical research industry is experiencing robust growth, driven by the increasing number of clinical trials and the need for innovative medical solutions. This growth translates into a strong job market and competitive salaries for clinical research professionals.
Trends in Clinical Research Salaries
Recent trends indicate that salaries in clinical research are on the rise. Factors contributing to this trend include:
Increased Funding for Research: Governments and private organizations are investing heavily in medical research, leading to higher salaries.
Technological Advancements: The integration of new technologies in clinical trials requires skilled professionals, driving up salaries.
Globalization of Clinical Trials: The expansion of clinical trials to new markets increases demand for qualified researchers worldwide.
Regional Salary Differences
Salaries for clinical research professionals can vary significantly by region. For example:
United States: Clinical researchers in the U.S. typically earn higher salaries compared to their counterparts in other countries due to the high demand and cost of living.
Europe: Salaries in Europe vary widely, with countries like Switzerland and Germany offering higher pay rates.
Asia: Emerging markets in Asia, such as China and India, are seeing rapid growth in clinical research, leading to competitive salaries.
Obtaining Advanced Certifications
Earning advanced certifications can significantly enhance your career prospects and salary potential. Key certifications include:
Certified Clinical Research Professional (CCRP): Offered by the Society of Clinical Research Associates (SOCRA).
Clinical Research Coordinator (CRC): Provided by the Association of Clinical Research Professionals (ACRP).
Clinical Data Manager (CDM): Certification through the Society for Clinical Data Management (SCDM).
Continuing Education and Training
Staying current with the latest developments in clinical research is crucial for career advancement. Consider the following options:
Advanced Degrees: Pursuing a master's or doctoral degree in clinical research or a related field.
Professional Development Courses: Enrolling in specialized courses to enhance specific skills.
Industry Conferences and Workshops: Attending events to network and stay informed about industry trends.
Conclusion
A career in clinical research offers not only the opportunity to contribute to groundbreaking medical advancements but also the potential for a lucrative salary. With the right training, certifications, and commitment to continuous learning, clinical research professionals can enjoy a rewarding and financially secure career.
By understanding the factors that influence salaries and taking proactive steps to enhance your qualifications, you can position yourself for success in this dynamic and growing field.
References
Exploring Non-Clinical Opportunities for Physicians
Understanding the Appeal of Non-Clinical Roles
The medical field has long been revered for its dedication to patient care and medical advancement. However, the traditional path of a physician often involves long hours, high stress, and significant personal sacrifice. In recent years, a growing number of physicians have sought alternative career paths that utilize their medical expertise while offering a different lifestyle. Non-clinical opportunities provide a viable option for those looking to transition out of direct patient care without leaving the medical field altogether. In this blog, we will explore various non-clinical roles available to physicians, discuss the benefits and challenges of these careers, and provide guidance on making the transition successfully.
Work-Life Balance
One of the most compelling reasons physicians seek non-clinical roles is the potential for improved work-life balance. Clinical practice often demands long hours, night shifts, and being on call, which can take a toll on personal life and health. Non-clinical roles typically offer more regular hours and greater flexibility.
Diverse Career Opportunities
Non-clinical careers span a wide range of industries, including pharmaceuticals, healthcare administration, medical writing, and consulting. These roles allow physicians to leverage their medical knowledge in new and exciting ways.
Continued Use of Medical Expertise
Many physicians worry that leaving clinical practice means abandoning their medical training. However, non-clinical roles often require a deep understanding of medical concepts and patient care, allowing physicians to continue utilizing their expertise.
Types of Non-Clinical Opportunities
Healthcare Administration
Healthcare administration is a popular choice for physicians seeking non-clinical roles. These positions involve overseeing healthcare facilities, managing budgets, and improving patient care delivery systems. Key roles include:
Hospital Administrator
Medical Director
Chief Medical Officer
Pharmaceutical and Biotechnology Industries
The pharmaceutical and biotechnology industries offer various roles for physicians, including:
Medical Science Liaison
Clinical Research Scientist
Regulatory Affairs Specialist
Medical Writing and Communications
Physicians with strong writing skills may find rewarding careers in medical writing and communications. These roles include:
Medical Writer
Medical Editor
Healthcare Communications Specialist
Consulting
Consulting provides an opportunity for physicians to offer their expertise to healthcare organizations, insurance companies, and other entities. Roles in this field include:
Healthcare Consultant
Management Consultant
Medical Advisor
Education and Training
Physicians can also transition into roles focused on education and training, such as:
Medical School Faculty
Continuing Medical Education (CME) Provider
Medical Training Program Director
Benefits and Challenges of Non-Clinical Careers
Benefits
Improved Work-Life Balance: Non-clinical roles often come with regular hours and greater flexibility, allowing for a better work-life balance.
Diverse Career Opportunities: The variety of non-clinical roles means physicians can find a position that matches their interests and skills.
Continued Use of Medical Expertise: Many non-clinical positions still require a strong medical background, allowing physicians to leverage their knowledge in new ways.
Challenges
Transitioning Skills: Moving from a clinical to a non-clinical role can be challenging, as it often requires developing new skills and knowledge.
Financial Considerations: Some non-clinical roles may offer lower salaries compared to clinical practice, although this varies widely.
Identity and Satisfaction: Physicians may struggle with the change in identity and job satisfaction when leaving direct patient care.
Making the Transition to a Non-Clinical Career
Assessing Your Interests and Skills
Before making a transition, it’s important to assess your interests and skills. Consider what aspects of your clinical work you enjoy and how they might translate to a non-clinical role. Reflect on your strengths and areas where you may need additional training or education.
Networking and Research
Networking is crucial when transitioning to a non-clinical career. Connect with colleagues who have made similar transitions, attend industry conferences, and join professional organizations. Research potential roles and employers to gain a better understanding of the opportunities available.
Gaining Additional Qualifications
Some non-clinical roles may require additional qualifications or certifications. For example, a physician looking to move into healthcare administration might benefit from earning a Master of Business Administration (MBA) or a Master of Health Administration (MHA).
Building a Strong Resume and Online Presence
A strong resume and online presence are essential when applying for non-clinical roles. Highlight your medical expertise, relevant skills, and any additional qualifications. Consider creating a LinkedIn profile to connect with industry professionals and showcase your experience.
Preparing for Interviews
Prepare for interviews by practicing responses to common questions and researching the company or organization. Be ready to discuss how your medical background and skills make you a good fit for the role. Emphasize your ability to adapt and learn new things quickly.
Key Considerations for Physicians Exploring Non-Clinical Careers
Financial Implications
Transitioning to a non-clinical career can have financial implications. While some non-clinical roles offer competitive salaries, others may pay less than clinical practice. It's important to evaluate your financial situation and consider how a change in salary might impact your lifestyle.
Emotional and Psychological Impact
Leaving clinical practice can be emotionally challenging. Physicians often identify strongly with their role in patient care, and transitioning to a non-clinical career can feel like a loss of identity. It's important to address these feelings and seek support from peers, mentors, or a professional counselor if needed.
Continuous Learning and Adaptation
Non-clinical roles may require continuous learning and adaptation. Physicians should be prepared to acquire new skills, stay updated on industry trends, and be open to new experiences. This commitment to ongoing professional development can enhance job satisfaction and career success.
Successful Examples of Physicians in Non-Clinical Roles
Dr. John Smith: Transitioned from clinical practice to a leadership role in healthcare administration, becoming a Chief Medical Officer at a major hospital.
Dr. Jane Doe: Moved into the pharmaceutical industry as a Medical Science Liaison, leveraging her medical expertise to bridge the gap between research and clinical practice.
Dr. Richard Roe: Pursued a career in medical writing, authoring research papers and medical textbooks that contribute to the advancement of medical knowledge.
Tips for a Smooth Transition
Seek Mentorship: Find a mentor who has successfully transitioned to a non-clinical role. Their guidance and insights can be invaluable.
Stay Connected: Maintain connections with your medical peers and professional networks. This can provide support and potential job leads.
Focus on Transferable Skills: Highlight transferable skills such as leadership, problem-solving, and communication in your job applications.
Be Patient: The transition to a non-clinical career may take time. Be patient and persistent in your job search and professional development efforts.
Conclusion
Exploring non-clinical opportunities can be a rewarding career path for physicians seeking to leverage their medical expertise in new ways while achieving a better work-life balance. With careful planning, networking, and a willingness to learn, physicians can successfully transition to non-clinical roles that offer both personal and professional fulfillment. By considering the benefits and challenges, assessing their interests and skills, and seeking mentorship, physicians can navigate this transition smoothly and find new ways to contribute to the healthcare industry.
References
American College of Healthcare Executives. (2023). Healthcare Administration Careers. Retrieved from ACHE
Pharmaceutical Research and Manufacturers of America. (2023). Careers in the Pharmaceutical Industry. Retrieved from PhRMA
American Medical Writers Association. (2023). Medical Writing as a Career. Retrieved from AMWA
Society of Healthcare Consultants. (2023). Consulting Careers for Physicians. Retrieved from SHC
American Association for Physician Leadership. (2023). Physician Leadership and Education. Retrieved from AAPL
Institute for Continuing Medical Education. (2023). Opportunities in CME. Retrieved from ICME
By following these guidelines and considering the various aspects of non-clinical careers, physicians can make informed decisions and find fulfilling roles that align with their professional goals and personal aspirations.
Clinical research remote positions
The Evolution of Remote Clinical Research
In recent years, the landscape of clinical research has seen a significant shift towards remote positions. This transformation is driven by advancements in technology, changing workplace dynamics, and the need for greater flexibility. In this blog, we will explore the key aspects of remote positions in clinical research, their benefits, challenges, and future prospects.
A New Era in Clinical Research
The COVID-19 pandemic accelerated the adoption of remote work across various industries, including clinical research. This shift has enabled clinical trials to continue with minimal disruption, ensuring that critical research can progress without compromising on safety or data integrity. Remote clinical research positions have become more common, allowing professionals to work from anywhere in the world.
Key Drivers of Remote Clinical Research
Several factors have contributed to the rise of remote positions in clinical research:
Technological Advancements: Improved communication tools, electronic data capture (EDC) systems, and remote monitoring technologies have made it easier to conduct clinical research from a distance.
Regulatory Flexibility: Regulatory bodies, such as the FDA, have provided guidance on decentralized clinical trials, paving the way for remote research methods.
Cost Efficiency: Remote work reduces overhead costs associated with maintaining physical office spaces and travel expenses.
Access to a Broader Talent Pool: Organizations can tap into a global talent pool, attracting skilled professionals from diverse geographic locations.
Benefits of Remote Positions in Clinical Research
Enhanced Flexibility and Work-Life Balance
Remote positions offer clinical research professionals the flexibility to work from home or any location of their choice. This flexibility enhances work-life balance, reducing stress and increasing job satisfaction.
Flexibility: Professionals can create their own schedules, leading to a better work-life balance.
Reduced Commute: Eliminating the daily commute saves time and money, contributing to a healthier lifestyle.
Increased Productivity: Many professionals find that they are more productive when working in a comfortable and familiar environment.
Access to Global Talent
Remote positions break down geographic barriers, allowing organizations to hire the best talent from around the world. This diversity brings fresh perspectives and innovative solutions to clinical research challenges.
Diverse Expertise: Access to a wider pool of candidates with varied expertise and backgrounds.
Global Collaboration: Enhanced collaboration across different time zones, leading to round-the-clock progress on research projects.
Competitive Advantage: Organizations can stay ahead of the competition by leveraging the skills and knowledge of a global workforce.
Challenges and Solutions in Remote Clinical Research
Overcoming Communication Barriers
Effective communication is crucial in remote clinical research. Without face-to-face interactions, it can be challenging to ensure clear and consistent communication among team members.
Regular Check-ins: Implement regular virtual meetings to keep everyone aligned and address any issues promptly.
Collaboration Tools: Utilize advanced collaboration tools, such as Slack, Microsoft Teams, and Zoom, to facilitate seamless communication.
Clear Documentation: Maintain thorough and accessible documentation to ensure everyone has the information they need.
Ensuring Data Security and Compliance
Remote work can pose challenges to data security and regulatory compliance. It is essential to implement robust measures to protect sensitive information and adhere to regulatory standards.
Secure Access: Use secure, encrypted connections for accessing and sharing data.
Training and Awareness: Provide regular training on data security best practices and regulatory requirements.
Compliance Monitoring: Implement continuous monitoring to ensure compliance with industry regulations.
Future Prospects of Remote Clinical Research Positions
Continued Growth and Innovation
The trend towards remote clinical research positions is expected to continue, driven by ongoing technological advancements and the need for flexible work arrangements. Organizations will increasingly adopt decentralized clinical trial models, leveraging remote monitoring and data collection technologies.
Embracing Hybrid Models
While remote work offers numerous benefits, some aspects of clinical research may still require in-person interactions. Hybrid models, combining remote and on-site work, will likely become more prevalent, offering the best of both worlds.
Investing in Remote Work Infrastructure
Organizations will need to invest in robust remote work infrastructure to support their teams effectively. This includes advanced communication tools, secure data management systems, and comprehensive training programs.
Conclusion
Remote positions in clinical research are transforming the industry, offering numerous benefits while presenting unique challenges. By embracing technological advancements and implementing effective communication and data security measures, organizations can successfully navigate this new landscape. The future of clinical research lies in the balance between remote and on-site work, ensuring flexibility, efficiency, and innovation.
For more insights and updates on clinical research trends, check out the following references:
FDA Guidance on Decentralized Clinical Trials
By staying informed and adapting to the evolving landscape, clinical research professionals can thrive in remote positions and contribute to groundbreaking advancements in medical science.
Clinical Trial Associate vs Clinical Research Associate
Understanding the Key Differences and Roles
Clinical research plays a crucial role in the advancement of medical knowledge and the development of new treatments and therapies. Within this field, the roles of Clinical Trial Associates (CTAs) and Clinical Research Associates (CRAs) are often discussed and sometimes confused. While both positions are vital to the success of clinical trials, they have distinct responsibilities and requirements.
In this comprehensive blog, we will explore the roles of CTAs and CRAs in depth, highlighting their differences, similarities, and the unique contributions each role makes to the clinical research process.
Clinical trials are the backbone of medical research, providing the necessary evidence to support new treatments, medications, and interventions. The success of these trials relies on a well-coordinated effort from various professionals, including Clinical Trial Associates (CTAs) and Clinical Research Associates (CRAs). Understanding the distinct roles and responsibilities of these positions is essential for anyone considering a career in clinical research or looking to collaborate effectively within a clinical trial team.
What is a Clinical Trial Associate (CTA)?
A Clinical Trial Associate (CTA) is a key member of the clinical trial team, responsible for providing administrative and operational support throughout the duration of a clinical study. CTAs are often involved in the early stages of the trial, assisting with the preparation and organization of essential documents and ensuring that all regulatory requirements are met.
Key Responsibilities of a CTA
Document Management: CTAs are responsible for the creation, maintenance, and organization of trial master files (TMFs), ensuring that all essential documents are properly filed and accessible.
Regulatory Compliance: They assist in the preparation and submission of regulatory documents, ensuring compliance with local, national, and international regulations. For more information on the roles and responsibilities, refer to the Clinical Trial Associate Job Description on Betterteam.
Communication: CTAs facilitate communication between the clinical trial team, study sites, and external stakeholders, ensuring that all parties are informed and updated on trial progress.
Administrative Support: They provide general administrative support, including scheduling meetings, preparing meeting minutes, and coordinating travel arrangements for the clinical trial team.
Data Entry and Management: CTAs may be involved in the entry and management of trial data, ensuring accuracy and completeness of data collected during the study.
Skills and Qualifications Required for a CTA
Educational Background: A bachelor's degree in life sciences, healthcare, or a related field is typically required. Some positions may accept candidates with an associate degree or relevant work experience.
Attention to Detail: CTAs must have a keen eye for detail, ensuring that all documents and data are accurate and complete.
Organizational Skills: Strong organizational skills are essential for managing multiple tasks and ensuring that all trial-related documents are properly maintained.
Communication Skills: Excellent written and verbal communication skills are necessary for effectively coordinating with study sites and other stakeholders.
Technical Proficiency: Proficiency in Microsoft Office and clinical trial management systems (CTMS) is often required. Additional insights can be found in the Roles and Responsibilities of a Clinical Trial Associate on JobHero.
What is a Clinical Research Associate (CRA)?
A Clinical Research Associate (CRA) plays a more hands-on role in the clinical trial process, primarily responsible for monitoring the progress of clinical studies and ensuring that they are conducted in accordance with regulatory requirements and study protocols. CRAs often work directly with study sites, conducting site visits and ensuring that data collected is accurate and complete.
Key Responsibilities of a CRA
Site Monitoring: CRAs conduct regular site visits to monitor the progress of clinical trials, ensuring that study sites adhere to the protocol and regulatory requirements. Detailed information about CRA roles can be found on ProClinical.
Data Verification: They review and verify data collected during the trial, ensuring its accuracy and completeness.
Training and Support: CRAs provide training and support to site staff, ensuring that they are knowledgeable about the study protocol and procedures.
Regulatory Compliance: They ensure that all regulatory requirements are met and that study sites are prepared for audits and inspections.
Issue Resolution: CRAs identify and resolve any issues or discrepancies that arise during the trial, ensuring that the study stays on track and within compliance.
Skills and Qualifications Required for a CRA
Educational Background: A bachelor's degree in life sciences, healthcare, or a related field is typically required. Advanced degrees or certifications (e.g., CCRA) can be advantageous. For further career guidance, see the Clinical Research Associate Career Profile on Study.com.
Clinical Experience: Prior experience in clinical research or a related field is often required, with many CRAs having a background in nursing, pharmacy, or other healthcare professions.
Analytical Skills: Strong analytical skills are essential for reviewing and verifying trial data.
Communication Skills: Excellent communication skills are necessary for effectively interacting with study sites and other stakeholders.
Travel Flexibility: CRAs often travel frequently for site visits, so flexibility and willingness to travel are important.
Differences Between CTAs and CRAs
While both CTAs and CRAs play critical roles in clinical trials, their responsibilities and focus areas differ significantly.
Scope of Work
CTAs: Primarily focused on administrative and operational support, ensuring that all documents and regulatory requirements are in place. Their work is often more office-based.
CRAs: Focused on site monitoring and data verification, ensuring that trials are conducted according to protocol and regulatory standards. Their work often involves significant travel and direct interaction with study sites. For more comparison details, visit Clinical Research Associate vs. Clinical Research Coordinator on Clinical Professionals.
Interaction with Study Sites
CTAs: Limited direct interaction with study sites, primarily facilitating communication and providing administrative support from a central location.
CRAs: Regularly interact with study sites, conducting site visits, providing training, and resolving issues directly with site staff.
Data Handling and Management
CTAs: May be involved in data entry and management, ensuring that data is accurately recorded and maintained.
CRAs: Responsible for verifying the accuracy and completeness of data collected during the trial, ensuring that it meets regulatory and protocol standards.
Similarities Between CTAs and CRAs
Despite their differences, CTAs and CRAs share some commonalities in their roles and contributions to clinical trials.
Contribution to Clinical Trials
Both CTAs and CRAs play essential roles in ensuring the success of clinical trials, contributing to the overall goal of advancing medical knowledge and improving patient care. For more on clinical trials, see Clinical Trials: What You Need to Know on the FDA website.
Regulatory Compliance
Both positions require a strong understanding of regulatory requirements and guidelines, ensuring that clinical trials are conducted ethically and in compliance with all applicable regulations.
Career Pathways for CTAs and CRAs
Both CTAs and CRAs have opportunities for career advancement and professional development within the field of clinical research.
Career Advancement Opportunities
CTAs: With experience and additional training, CTAs can advance to positions such as Clinical Research Coordinator (CRC), Regulatory Affairs Specialist, or Project Manager.
CRAs: CRAs can advance to senior CRA positions, Lead CRA, Clinical Project Manager, or Clinical Operations Manager. For career guidance, refer to How to Become a Clinical Research Associate on WikiHow.
Professional Development
Both CTAs and CRAs can benefit from continued education and certification programs, such as the Clinical Research Coordinator (CRC) certification for CTAs or the Certified Clinical Research Associate (CCRA) certification for CRAs. For more on training and certifications, see Clinical Research Training and Certification on the AACR website.
Final Thoughts
The roles of Clinical Trial Associates (CTAs) and Clinical Research Associates (CRAs) are both vital to the success of clinical trials, each contributing in unique ways to the advancement of medical research. While CTAs provide essential administrative and operational support, ensuring that all regulatory requirements are met, CRAs take on a more hands-on role, monitoring study sites and verifying data accuracy. Understanding the distinct responsibilities and requirements of these roles is essential for anyone considering a career in clinical research or looking to collaborate effectively within a clinical trial team.
In the dynamic and ever-evolving field of clinical research, both CTAs and CRAs have the opportunity to make significant contributions to the development of new treatments and therapies, ultimately improving patient care and advancing medical knowledge.