CCRPS Course DATA
CCRPS Course covers double to triple the amount of course content than other courses. While many courses are simply 5-20 simple interactive modules, our course covers 140 dense modules in thorough detail. After each session, students can ask their questions privately with the course instructor, all of whom have 15+ years of CRA experience. We offer clinical research courses distance learning.
There is a huge shortage of well-trained CRAs, but many companies are reluctant to hire untrained entry-level clinical monitors because of patient and trial safety. Because of this, even the beginner entry-level jobs require certification or training. Most courses provide very light training that may look good because of the company names, but alone is not sufficient to pass the interview rounds a company conducts. Because we prepared our modules to stand by you even as a Senior Clinical Research Associate, we find more of our students with no background quickly passing their interview rounds. Our program is considered one of the top clinical research graduate programs online.
Currently, 82% of our students are hired within the first month of taking the course. Students with limited background or those looking to gain extra experience are offered a remote internship of up to 6 months during the time they are interviewing. This advantage allows many students with limited experience to get hired with a higher paying job than previously offered. For exam, nurses are offered our clinical research nurse training and can additionally take our clinical research management training.
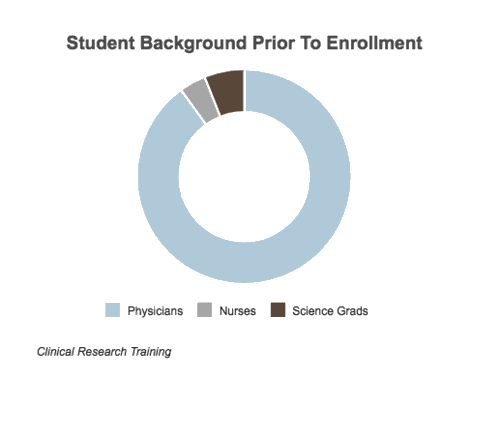
While a majority of our students are physicians, a majority of the CRA workforce are Science Grads and Nurses. We train at a Senior CRA level for all students regardless of background because clinical research monitoring is vastly different from any lab or science course you may have taken. Clinical research associates are given the protocol of a study including all medical protocol that must be followed but because they do not diagnose or treat, the medical knowledge is supplemental but not sufficient in this career path. This is the main reason why our Clinical Research Training includes all possible scenarios you may face at the protocol and guideline level in your future company.
CLINICAL RESEARCH ASSOCIATE TRAINING AND PLACEMENT
Learn about our partnerships with dedicated clinical research recruiters by inquiring. We provide an 8 module job application preparation section in our CRA certification course.
Always use a cover letter specific for the company and job when applying if you are not using a recruiter.