ICH GCP COURSE - ICH GCP CERTIFICATION
The Ultimate Guide to ICH GCP Certification
Understanding ICH GCP Certification
What is ICH?
The term ICH stands for the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. This global body develops guidelines to unify regulations for the design, execution, and quality assurance of clinical trials. Among its key contributions is the establishment of Good Clinical Practice (GCP) standards.
What is GCP?
GCP is an internationally recognized quality standard that governs clinical trials. It ensures that trials are conducted consistently, ethically, and scientifically. GCP guidelines prioritize two objectives:
Protecting Human Subjects: Ensuring the safety, welfare, and rights of research participants.
Guaranteeing Reliable Results: Ensuring data from clinical trials are accurate, credible, and usable for advancing medical science.
GCP is crucial for scientists, organizations, and professionals globally as it standardizes processes, ensuring that clinical trials are both ethical and trustworthy.
Why is ICH GCP Certification Necessary?
Obtaining an ICH GCP certification demonstrates a professional’s mastery of these global standards. It’s more than a certificate; it’s industry recognition of your ability to uphold the highest ethical and scientific standards in clinical research.
Who Needs ICH GCP Certification?
Professionals from various roles in the clinical research ecosystem benefit significantly from ICH GCP training and certification.
Steps to GCP Certification
NIH GCP Training: A foundational program provided by the National Institutes of Health (NIH) for researchers involved in clinical trials. This free course emphasizes the ethical principles of Good Clinical Practice and compliance with federal regulations. It includes practical guidelines for informed consent, patient safety, and data integrity, making it an excellent starting point for those entering the field.
Good Clinical Practice (GCP) Courses: These programs are tailored to educate clinical research professionals on the principles of GCP as outlined by ICH guidelines. They cover essential topics such as protocol development, trial management, and regulatory compliance. Many courses offer certifications upon completion, enhancing the knowledge and credibility of professionals across all roles in clinical trials.
CGCP Certification: The Certified Good Clinical Practice (CGCP) certification is designed for professionals looking to validate their expertise in clinical research ethics, trial safety, and global regulatory standards. This credential signals a deep understanding of GCP to employers, providing an edge in career advancement. It’s especially beneficial for CRAs, CRCs, and project managers aiming to solidify their roles in the industry.
Why These Support ICH GCP Guidelines
Reinforces Core Knowledge: All three options align with and complement the ICH GCP guidelines, ensuring that professionals fully understand compliance and safety requirements.
Tailored for Career Growth: They provide tailored training for beginners and experienced professionals alike, focusing on competencies critical for roles such as CRAs, investigators, and managers.
Global and Ethical Perspective: By emphasizing global standards, regulatory awareness, and the ethical conduct of trials, these certifications and courses prepare individuals for the evolving landscape of clinical research.
Enrolling in an ICH GCP certification course is a straightforward process, but choosing the right program is essential. CCRPS offers a trusted, comprehensive program tailored to your success.
ICH GCP Guidelines
ICH E6(R3): Focuses on decentralized clinical trials, risk-based monitoring, patient-centric designs, and scalable trial principles. This latest revision modernizes Good Clinical Practice (GCP) for global and digital research, with full adoption expected by 2025.
ICH E6(R2): Introduced in 2016, it includes risk-based monitoring, sponsor oversight, and CRO responsibilities. This version remains fundamental until E6(R3) is fully implemented.
ICH E8(R1): Promotes a quality-by-design framework, patient involvement, and reduced trial complexity. Revised in 2019 to incorporate adaptive and decentralized trial models.
ICH E2A: Sets definitions and processes for expedited reporting of adverse events, providing standardization across safety data management.
ICH E2B(R3): Establishes harmonized formats for electronic reporting of individual case safety reports (ICSRs). Updated to align with global pharmacovigilance systems like EudraVigilance.
ICH E3: Provides a standardized structure for clinical study reports, streamlining submissions to global regulatory agencies.
ICH E7: Offers guidance for trials involving geriatric populations, addressing aging-related conditions and comorbidities.
ICH E9(R1): Revised in 2019, it defines statistical principles such as estimands and sensitivity analysis, improving the clarity of trial objectives.
ICH E10: Advises on choosing ethical and scientifically sound control groups, including placebo, active comparators, or historical controls.
ICH M1: Maintains MedDRA, a standardized terminology for adverse event reporting, with regular updates to ensure clarity and consistency.
ICH M3(R2): Details timing and requirements for non-clinical safety studies preceding human clinical trials, aiming to streamline safety assessments.
ICH Q8(R2): Introduces a quality-by-design approach for pharmaceutical development, aligning with risk-based monitoring practices.
ICH Q9: Outlines principles for risk assessment, control, and communication, ensuring a systematic approach to managing quality risks in clinical research.
ICH Q10: Defines pharmaceutical quality systems and their application within clinical trials to maintain compliance and safety.
ICH S6(R1): Specifies safety evaluation criteria for biotechnology-derived pharmaceuticals, incorporating advances in biotechnology.
ICH E11(R1): Revised to improve methodologies for pediatric trials, emphasizing safety and pharmacokinetics tailored to younger populations.
ICH S9: Sets non-clinical safety standards for anticancer drugs, supporting quicker transitions to early-phase clinical trials.
Updates to Note
ICH E6(R3): Innovates to meet the demands of decentralized trials and advanced digital technologies like wearables and eConsent systems.
ICH E8(R1): Elevates patient-centric trials, reducing participant burdens and simplifying designs.
ICH E9(R1): Refines statistical standards to align trial objectives with therapeutic goals.
What Makes CCRPS GCP Training Stand Out?
Flexible, Online Learning: Courses are available entirely online for self-paced completion.
Up-to-Date Curriculum: Courses are compliant with the latest ICH GCP 2025 guidelines.
Accreditation: CCRPS certifications are accredited by the Accreditation Council for Clinical Research & Education (ACCRE).
Affordable: Accessible, cost-effective, and designed for professionals at different levels of experience.
Comprehensive Resources: Modules include real-world examples to enhance your learning.
Benefits of ICH GCP Certification
For Professionals:
Career Advancement: Certification enhances your resume and qualifies you for advanced roles in clinical research.
Skill Development: Gain a strong foundation in ethical and operational aspects of clinical trials.
Global Recognition: Show your expertise and credibility to employers globally.
For Employers:
Regulatory Compliance: Certified staff ensure trials meet legal and ethical standards.
Improved Performance: Certified teams run trials more efficiently and accurately.
Data Credibility: Boosts the reliability of trial results submitted to regulators.
BenefitHow It Adds ValueCareer OpportunitiesIncreased employability in global pharmaceutical, biotech, and CROs sectors.Global Compliance AwarenessPrepares you for international trials with harmonized procedures.Ethical AssuranceEnsures you can safeguard trial participants while meeting regulatory mandates.Improved CollaborationUnified language and processes improve communication with global research teams.
Learning Outcomes
By completing CCRPS’s GCP certification course, participants gain critical skills and knowledge, including:
The fundamentals of Good Clinical Practice and how to apply them in trials.
Strategies for designing and conducting scientifically robust and ethical trials.
Insights into monitoring, auditing, and reporting for both pre- and post-market clinical studies.
A thorough understanding of safeguarding participant rights and ensuring high-quality data integrity.
Additional Resources for ICH GCP Certification
Here are some notable resources to guide your learning and offer additional context:
ICH GCP Guidelines Official Texts: Review the full set of GCP principles to better grasp their application.
ClinicalTrials.gov: A catalog of trials adhering to GCP standards around the globe.
CCRPS Study Resources: Access in-depth modules, videos, and live assistance to prepare for your certification.
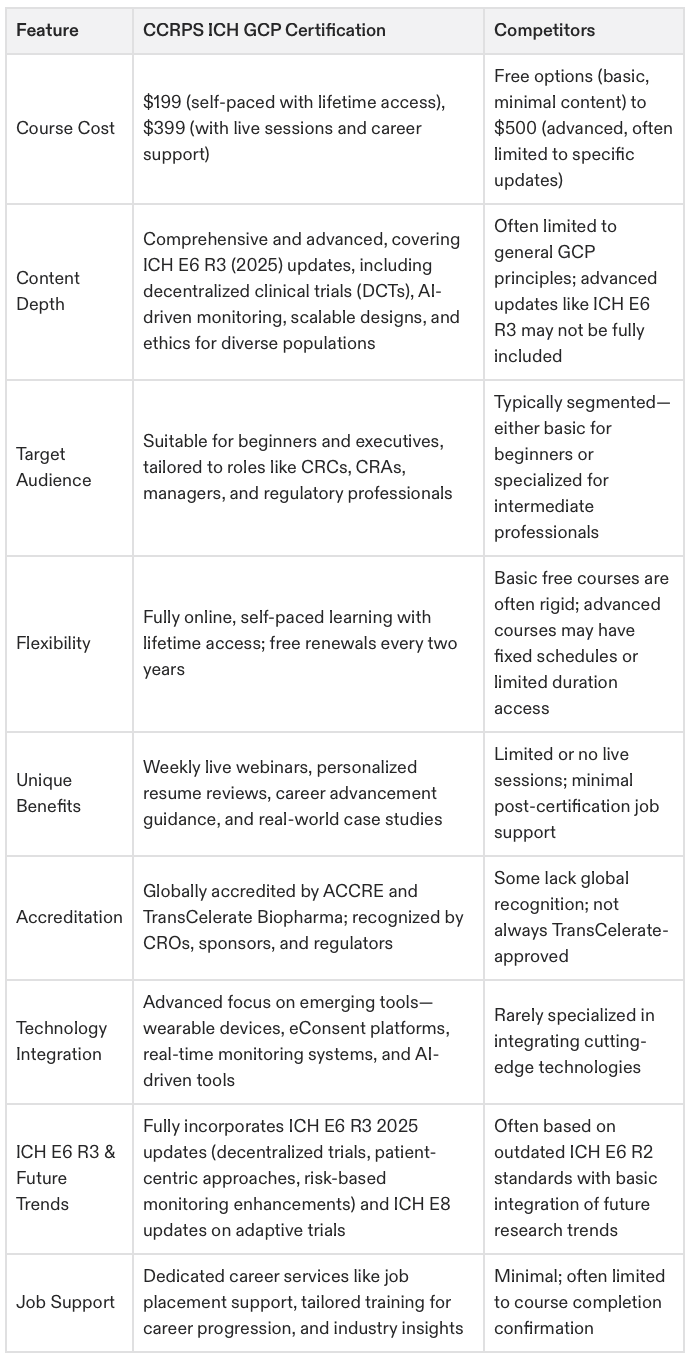
Why CCRPS is the Most Advanced Option for Clinical Research Professionals
Designed for All Levels: Whether you're a beginner or an executive, CCRPS provides robust training to accelerate your career in clinical research.
Future-Proof Curriculum: Fully updated for ICH E6 R3 2025, the program equips you with skills for modern challenges like decentralized trials and AI-enhanced data monitoring.
Lifetime Access at $199: Unlike competitors charging up to $500 for single-use courses, CCRPS offers lifetime access and free renewals to ensure you stay compliant at no extra cost.
Real-World Applications: Includes advanced case studies and role-specific responsibilities, ensuring you're well-prepared for diverse challenges in clinical research.
Career Growth Support: Weekly live webinars and personalized mentoring set you apart, supporting long-term success in roles at every level, from CRA to VP of Clinical Operations.
Globally Recognized Accreditation: Certified by ACCRE and TransCelerate Biopharma, trusted by over 3,000 professionals in clinical research globally.
Take your clinical research career to the next level—enroll in the CCRPS ICH GCP Certification today to gain top-tier training and unlock unparalleled career opportunities.
Frequently Asked Questions
1. Who Should Take the CCRPS Certification Course?
Anyone working in clinical trials or planning to enter the field will find value in this globally recognized certification.
2. How Long Does the Program Take?
Modules are self-paced and can be completed within 2-7 days.
3. Is the CCRPS Course Accredited?
Yes, it is accredited by the Accreditation Council for Clinical Research & Education (ACCRE) and meets the latest ICH GCP guidelines.
4. What If I Need Support During the Course?
CCRPS offers live assistance and a library of resources to ensure success.
Begin Your Journey Today
The demand for certified professionals in clinical research is at an all-time high. By earning your ICH GCP Certification through CCRPS, you’ll meet these demands head-on with the skills, confidence, and credentials to thrive in a competitive industry.
Start your certification now and position yourself as a leader in global clinical trials. Whether you’re looking to enhance your career, ensure trial compliance, or become a trusted figure in medical research, CCRPS.org is your partner in professional success.
Equip yourself with the knowledge, tools, and global expertise to excel. Enroll in the ICH GCP Certification course today!