Albania Clinical Research Work Guide
Welcome to the wild world of Clinical Research in Albania, where the stethoscopes are savvy, and the lab coats get their own LinkedIn profiles! If you thought clinical research was all about serious faces and sterile environments, think again. Albania is turning the tables, or should we say, test tubes, on what it means to be a Clinical Research Associate (CRA). Strap in, because you're about to dive into a career that's as thrilling as navigating the twists and turns of the Albanian Riviera—only with more data and fewer sunburns.
Why Clinical Research in Albania?
Albania has opened its arms wide to healthcare innovations and research. The government has also invested more on healthcare infrastructure which has facilitated better and advanced clinical trials and research projects. Furthermore, there are more and more collaborations with international pharmaceutical companies, which makes Albania not only a follower, but a leader in clinical research.
A Day in the Life of a CRA in Albania
Visualize starting your day with a sight of the Adriatic Sea, drinking Albanian coffee and getting ready for the day that you are going to make a difference. CRAs in Albania are in the middle of a dynamic work environment, which includes monitoring clinical trials, guaranteeing that they are compliant with the regulatory requirements and thus helping in the safe development of new therapies.
Daily Responsibilities of a CRA in Albania
1. Site Monitoring:
Site monitoring is a core function of a CRA and involves several critical tasks:
Protocol Compliance: CRAs make sure that every part of the clinical trial is in accordance with the pre-defined protocols which are meant to facilitate the testing of the medical hypotheses without putting the patients' health at risk. It includes tasks like verifying whether the staff of the site is complying with the rules of patient selection, treatment dosages and the time of the trial.
Safety Monitoring: A major concern of the CRA is patient safety. It includes routine screening for adverse events or side effects experienced by participants. CRAs spend much time with site staff to make sure that all the potential risks can be recognized, reported, and solved properly, as required by the regulations.
Quality Control: Another critical task is to ensure that data collected by clinical sites are accurate and verifiable. CRAs review source documents, compare them with case report forms (CRFs), and correct discrepancies to maintain the integrity of the trial's data.
2. Data Handling:
Effective data handling is crucial for the success of clinical research. This responsibility covers several key activities:
Data Collection: CRAs collect data from clinical trials, ensuring that every piece of information is accurately captured and recorded. This includes patient data from medical tests, treatment results, and follow-up visits.
Data Analysis: While CRAs are not always directly involved in the in-depth statistical analysis, they do need to have an understanding of how to interpret data trends and outcomes. This competency helps them ensure the data makes logical sense and supports the clinical objectives.
Data Management: CRAs ensure that data is not only collected and analyzed but also securely managed and stored. They use various electronic data capture (EDC) systems to enter data, maintain databases, and ensure that all information complies with regulatory requirements like GDPR in Europe or HIPAA in the United States.
3. Stakeholder Communication:
Effective communication is vital in the role of a CRA, as they act as the bridge between the research site and various stakeholders:
Regular Updates: CRAs regularly update all stakeholders on the progress of the trial. This includes internal teams, external clients or sponsors, and sometimes even regulatory bodies.
Problem Solving: When issues arise, whether with protocol compliance, patient safety, or data integrity, CRAs communicate these challenges to the appropriate parties. They then collaborate to find solutions that adhere to regulatory standards and ethical considerations.
Training and Support: CRAs often provide ongoing support and training to clinical site staff to ensure compliance with the clinical trial protocol and regulatory requirements. This might involve training new staff on proper documentation practices or updating teams on revised regulatory guidelines.
Through these responsibilities, CRAs in Albania and beyond ensure that clinical trials are conducted efficiently, ethically, and in compliance with all necessary regulations. This not only protects the welfare of participants but also ensures the credibility of the trial results, which are critical for medical advancements.
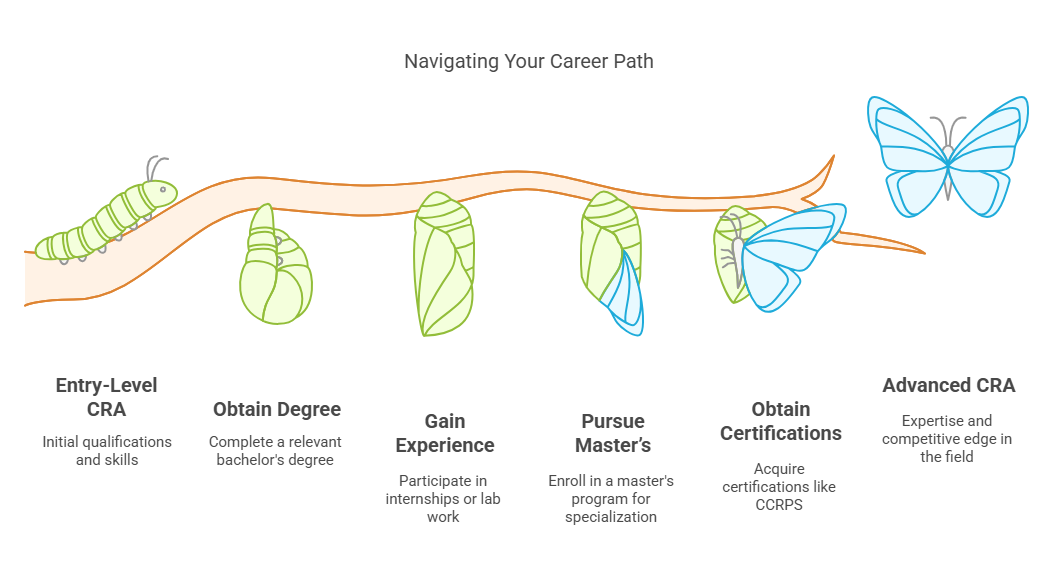
Navigating Your Career Path
Navigating your career as a Clinical Research Associate (CRA) in Albania involves deliberate choices and a commitment to educational excellence. The path to becoming a CRA is structured yet dynamic, offering numerous opportunities for advancement and specialization. Let's delve deeper into the educational pathways and the importance of certifications and further education in shaping a successful career in this field.
Educational Pathways
Bachelor’s Degree: The foundational step in becoming a CRA is to obtain a bachelor's degree in a relevant field. Degrees in life sciences, nursing, or pharmacy are particularly valuable because they provide the scientific background necessary for understanding clinical trials' complexities.
Life Sciences: Degrees in biology, biochemistry, or biotechnology offer comprehensive knowledge of the biological systems and processes that are critical in clinical research.
Nursing: A nursing degree equips candidates with hands-on patient care skills, which are invaluable when monitoring clinical trials and ensuring participant safety.
Pharmacy: A pharmacy degree provides in-depth knowledge of drug development and pharmacology, essential for managing and overseeing the medication-related aspects of clinical trials.
Academic Institutions: In Albania, universities in Tirana and other major cities such as Durrës and Vlorë offer programs tailored to these fields. These programs often include:
Coursework: Fundamental courses cover anatomy, physiology, pharmacology, and biostatistics, providing a solid scientific base.
Practical Experiences: Many programs incorporate practical training, such as internships or lab work, which are crucial for gaining hands-on experience in the clinical research environment.
Certifications and Further Education
After completing a bachelor’s degree, furthering your education through certifications or a master’s degree can significantly enhance your career prospects and earning potential.
Master’s Degree: Pursuing a master's degree in clinical research, public health, or a related field can deepen your expertise and make you more competitive in the job market.
Specialized Knowledge: Advanced degrees offer more specialized knowledge in areas like clinical trial management, regulatory affairs, and ethical considerations.
Research Opportunities: These programs often involve a significant research component, allowing students to contribute to ongoing studies, which can enhance their understanding and visibility in the field.
Certifications: Certifications provide practical, role-specific training that can be pivotal in securing a position as a CRA.
CCRPS Certification: The Certified Clinical Research Professional (CCRPS) certification is one example that is recognized internationally and provides training on the essential aspects of clinical trial conduct, GCP (Good Clinical Practice), and regulatory compliance.
Other Certifications: Other certifications might focus on project management, data management, or specific regulatory environments, all of which are valuable to a CRA.
Advantages of Advanced Education and Certifications:
Increased Job Opportunities: Higher qualifications often lead to more job opportunities, including leadership roles within clinical research projects.
Higher Salary Potential: As you increase your qualifications, you generally increase your earning potential as well. Employers value advanced education and specialized training, which is often reflected in salary offerings.
Professional Credibility: Advanced degrees and certifications can also enhance your professional credibility, making you a more attractive candidate for employers and a respected colleague within the scientific community.
By carefully selecting your educational and certification paths, you can significantly impact your career development as a CRA in Albania. This strategic approach not only prepares you for the diverse demands of clinical research but also positions you well for future advancements.
Salaries Unveiled: What Can You Really Earn?
In the clinical research industry in Albania, salary levels are influenced by a variety of factors including educational background, level of experience, and the specific employer. Understanding the salary structure for Clinical Research Associates (CRAs) is crucial for those considering this career path, as it provides insight into potential financial progression and motivation. Here’s a more detailed exploration of what CRAs in Albania can expect to earn at different stages of their careers:
Entry-Level Salaries
Starting Point: Entry-level CRAs typically begin their careers soon after completing a university degree. At this stage, the salary reflects the foundational skills and knowledge gained through education but limited practical experience.
Expected Salary: The starting salary for a new CRA in Albania is around 60,000 ALL per month. This competitive entry-level wage is designed to attract skilled graduates who can handle the responsibilities of monitoring clinical trials, managing data, and ensuring compliance with regulatory standards.
Factors Influencing Start Salary: The initial salary can vary depending on the specific sector (public vs. private), the size and scope of the employer (local clinics vs. international pharmaceutical companies), and the nature of the clinical trials they will be overseeing.
Mid-Career Salaries
Growing Expertise: As CRAs gain experience, their value to employers increases significantly. This is reflected in their salary progression.
Experience Range: This stage covers professionals who have been in the field for 2 to 5 years. It's a critical period where CRAs expand their skills through hands-on experience, including more complex studies and potentially larger, multi-site trials.
Expected Salary: Mid-career CRAs can expect a salary of approximately 80,100 ALL per month. The 34% increase from the entry-level salary acknowledges the added value brought by their deeper understanding of clinical protocols, enhanced data management capabilities, and improved stakeholder communication.
Additional Benefits: Besides salary, mid-career CRAs may begin to receive additional benefits such as bonuses, higher travel allowances, and professional development opportunities, reflecting their increased contribution to research projects.
Senior-Level Salaries
Advanced Expertise and Leadership: Senior CRAs have substantial experience and often take on leadership roles in clinical research projects. Their comprehensive expertise and professional networks significantly enhance their earning potential.
Experience Range: This category includes CRAs with 5 to 10 years of experience or more. At this level, professionals often oversee entire research programs or multiple projects and might also be involved in strategic planning and regulatory submissions.
Expected Salary: Salaries for senior-level CRAs can reach around 118,000 ALL per month. This reflects their critical role in ensuring the scientific and ethical integrity of clinical research, as well as their capacity to mentor less experienced staff and manage complex logistical challenges.
Factors Influencing Senior Salary: At this level, other factors such as previous successful trial outcomes, specialized knowledge in high-demand therapeutic areas, and additional qualifications such as a master's degree or specialized certifications can further influence salary levels.
Link: https://worldsalaries.com/average-clinical-research-associate-salary-in-albania/
Explore Courses for Clinical Research Careers in Albania
Courses Available:
Conclusion
Embarking on a career as a Clinical Research Associate (CRA) in Albania offers a unique opportunity to contribute to the advancement of medical science while building a rewarding professional life. With the right qualifications, a commitment to continuous learning, and an understanding of the evolving landscape of clinical research, aspiring CRAs can look forward to a promising future.
Whether you're just starting out or looking to advance your career, CCRPS is here to support you every step of the way. Our comprehensive training programs, resources, and networking opportunities are designed to equip you with the skills and knowledge needed to succeed in this dynamic field.
Frequently Asked Questions (FAQs)
-
To become a CRA in Albania, you typically need a bachelor’s degree in life sciences, nursing, or pharmacy. Additionally, possessing certifications in clinical research, such as those from CCRPS or ACRP, can enhance your prospects. A strong understanding of Good Clinical Practices (GCP) and regulatory requirements is also essential.
-
Salaries for CRAs in Albania are generally competitive within the Balkan region, though they may be lower compared to EU countries. In neighboring countries like Greece or Bulgaria, salaries might be slightly higher due to larger market sizes and more international company presence.
-
Career advancement for CRAs in Albania can include transitioning to senior CRA roles, becoming project managers, or specializing in specific areas of clinical research such as oncology or pediatric studies. Further education and certifications can also lead to roles in regulatory affairs or clinical operations management.
-
The daily work life of a CRA in Albania involves monitoring clinical trial sites, ensuring compliance with study protocols, managing study data, and liaising with trial sponsors and stakeholders. CRAs often travel to multiple sites and need to maintain detailed reports of their findings and communications.
-
Networking in the clinical research field in Albania can be enhanced by joining professional organizations, attending industry conferences, participating in workshops, and connecting with peers on LinkedIn. Building relationships with university professors and other healthcare professionals can also be beneficial.
-
Emerging trends in clinical research in Albania include the increasing use of digital technologies for data management and remote monitoring, a growing focus on patient-centric trials, and collaboration with international research organizations to conduct more global studies.
-
Further education can significantly impact your CRA career in Albania by qualifying you for higher-level positions, increasing your earning potential, and expanding your expertise in specialized areas of clinical research. Advanced degrees or specialized certifications can set you apart in the job market.
-
CCRPS provides comprehensive training and certification programs that cover essential aspects of clinical research, including study design, regulatory compliance, and ethical considerations. CCRPS also offers resources and support for continuing education and career development.
-
Begin by obtaining a relevant degree in life sciences, nursing, or pharmacy. Gain exposure through internships or related healthcare positions. Acquiring a certification in clinical research, such as from CCRPS, can further validate your expertise and readiness for CRA roles.
-
As you gain experience, you can expect salary increases of approximately 20-50% over several years. Entry-level CRAs may start around 60,000 ALL per month, with potential to reach over 100,000 ALL as a senior CRA, depending on the complexity of the trials and your level of responsibility.
-
Work-life balance can be challenging due to the travel and demands of trial monitoring. However, many employers offer flexible working arrangements, and effective time management can help. Seasoned CRAs often share that setting clear boundaries and prioritizing tasks effectively are key to maintaining balance.
-
In Albania, CRAs are particularly in demand in the pharmaceutical, biotechnology, and medical device industries. The demand is also growing in academic research centers and contract research organizations (CROs) that conduct trials on behalf of these industries.
-
CRAs should be proficient with Clinical Trial Management Systems (CTMS), Electronic Data Capture (EDC) systems, and patient management software. Familiarity with Microsoft Office and statistical software is also beneficial for managing and analyzing clinical data.
-
Yes, international experience can be highly beneficial, exposing you to a wider range of clinical practices and regulatory environments. It enhances your resume and can provide a competitive edge in the local market where global trial experience is highly valued.