Decentralized Clinical Trials A Revolution in Healthcare Research and the Evolving Role of CROs
Welcome to the future where clinical trials zip around your lifestyle like drones delivering your favorite pizza! Gone are the days of clinical trials confined to stuffy hospital rooms. Welcome to 2025, where Decentralized Clinical Trials (DCTs) and Contract Research Organizations (CROs) are not just buzzwords but the superheroes of healthcare research. Buckle up as we embark on a thrilling ride through this revolutionary landscape!
The Evolution of Decentralized Clinical Trials in 2025
A Patient-Centric Revolution In 2025, the adoption of Decentralized Clinical Trials (DCTs) has skyrocketed, transforming the fabric of medical research with a robust digital backbone. This seismic shift is powered by cutting-edge technologies and an unwavering commitment to patient convenience, turning any location into a potential trial site—from cozy living rooms to buzzing community centers.
Technological Transformation and Patient Convenience
Mainstreaming of DCTs: By 2025, DCTs have established themselves as a central methodology in conducting medical research. This shift is predominantly driven by rapid advancements in digital technology, which have matured enough to support complex and sensitive activities like clinical trials.
Expansion Beyond Traditional Settings: Traditionally, clinical trials have been confined to specific, controlled environments such as hospitals or specialized research facilities. However, with the advent of robust digital technologies, these trials can now be administered beyond these traditional settings. DCTs utilize digital platforms that enable the conduct of trials in more flexible and participant-friendly environments such as the participants' homes or local community centers.
Utilization of Digital Platforms: Digital platforms are at the heart of this transformation. These platforms can include a variety of technologies such as:
Telemedicine systems that allow for remote consultations and check-ins between patients and researchers or healthcare providers.
Mobile health applications that participants can use to report outcomes, manage consent forms, or receive reminders and instructions related to the trial.
Wearable devices that continuously gather health data such as heart rate, blood pressure, or activity levels, providing real-time insights into the participant’s health status without requiring in-person visits.
Enhanced Accessibility and Convenience: The use of these digital platforms means that participants no longer need to travel to research facilities, which can be time-consuming and disruptive. Instead, they can partake in clinical trials from the comfort of their own homes or a convenient local setting. This significantly lowers the barriers to participation, such as travel difficulties, time constraints, and the costs associated with frequent visits to trial sites.
Increased Participant Willingness: When clinical trials are less disruptive to everyday life, people are more likely to consider participating. This increased convenience boosts recruitment rates and widens the demographic diversity of trial participants. More diverse participant pools lead to more comprehensive data, which can enhance the generalizability of the trial results.
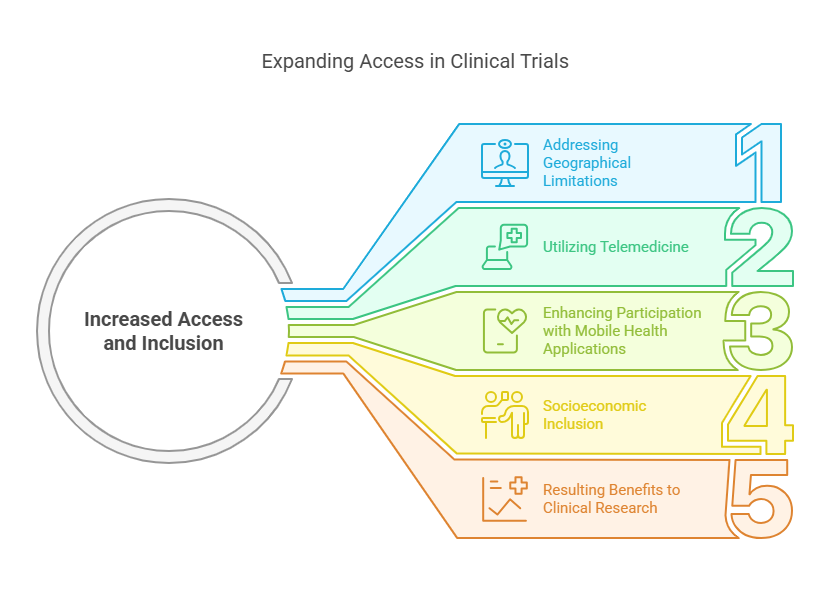
Increased Access and Inclusion
Addressing Geographical Limitations: Traditional clinical trials often require participants to visit specific sites, such as hospitals or research facilities, which can be located in urban centers or regions not easily accessible to everyone. This geographical barrier can discourage or outright prevent many potential participants, especially those living in remote or rural areas, from joining clinical trials. DCTs, utilizing telemedicine, enable these individuals to participate remotely, effectively removing the physical distance barrier.
Utilizing Telemedicine: Telemedicine involves the use of telecommunications technology to provide healthcare services remotely. In the context of DCTs, telemedicine allows for patient consultations, monitoring, and even certain procedural follow-ups to be conducted via online platforms. This means participants can engage in trial activities from their homes or local community health centers without the need to travel.
Enhancing Participation with Mobile Health Applications: Mobile health applications are another crucial tool in DCTs. These apps can be installed on smartphones or tablets and are designed to perform a variety of functions, such as:
Data Collection: Apps can collect health data entered by the user or synced from wearable devices.
Communication: They provide a direct line of communication between participants and trial coordinators, facilitating better and more immediate support.
Education and Engagement: Apps can deliver educational materials about the trial, remind participants of their schedule, or prompt them to take medications or complete trial-related tasks.
Socioeconomic Inclusion: Beyond geography, socioeconomic factors often influence who can participate in clinical trials. The costs associated with traveling to trial sites, taking time off work, or arranging childcare can exclude lower-income individuals. DCTs minimize or eliminate many of these costs, making participation feasible for a wider socio-economic group.
Resulting Benefits to Clinical Research: By breaking down these barriers, DCTs achieve a higher degree of inclusivity, which is vital for several reasons:
Diverse Data Sets: More varied demographic participation leads to data that more accurately reflects the general population. This diversity is crucial for understanding how treatments work across different groups, leading to safer and more effective therapies.
Improved Generalizability: With a broader range of participants, the results of clinical trials can be generalized to a larger population, enhancing the reliability of the research outcomes.
Ethical Research Practices: Ensuring that everyone has an opportunity to participate in clinical research promotes equity in healthcare advancements and supports ethical standards in medical research.
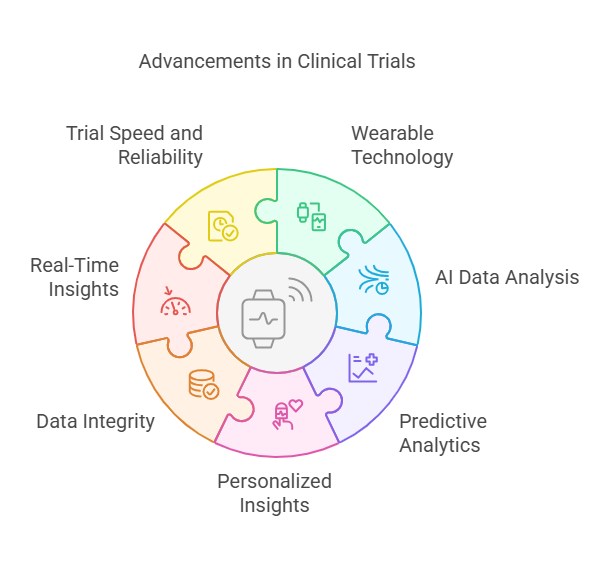
Enhanced Data Integrity and Real-Time Insights
Integration of Wearable Technology: Wearable devices are a cornerstone of innovation in DCTs. These devices, such as fitness trackers, smartwatches, and health monitors, are equipped with sensors that continuously gather a wide range of health metrics from participants. The types of data these wearables can collect include:
Heart rate and rhythm
Blood pressure
Activity levels and sleep patterns
Oxygen saturation
Glucose levels in diabetic patients
This data is collected in real time and transmitted to researchers or healthcare providers, allowing for continuous monitoring without the need for constant physical presence at a healthcare facility.
Role of Artificial Intelligence (AI): AI plays a transformative role in how this data is handled and analyzed. AI algorithms can process vast amounts of data much faster and more accurately than humanly possible. The capabilities of AI in DCTs include:
Data Analysis: AI can quickly identify patterns or anomalies in the data that may indicate important health insights or potential complications, often before they become evident to the patient or doctor.
Predictive Analytics: AI models can predict health outcomes based on historical and real-time data. This predictive capability is crucial for proactive management of conditions and can lead to interventions that prevent worsening health situations.
Personalized Insights: AI can tailor recommendations or treatments to individual patients based on their specific data, enhancing the personalization of care, which is a key goal in modern medicine.
Enhanced Data Integrity: The use of these technologies ensures that the data collected is both precise and reliable. Data integrity is critical in clinical trials as it directly affects the validity of the trial results. Real-time data collection reduces the lag between data acquisition and analysis, minimizing the risk of data degradation or loss that can occur with more traditional methods of data collection.
Real-Time Insights for Swift Adjustments: One of the most significant advantages of using wearable technology and AI in DCTs is the capability to obtain real-time insights. This immediacy allows trial supervisors to make swift adjustments to the trial protocol if needed, such as modifying dosages or treatment plans in response to how participants are reacting to a treatment. This agility can improve the safety of trials by quickly addressing any adverse reactions or complications.
Improving Trial Speed and Reliability: Finally, the real-time nature of data collection and analysis not only enhances the quality of the data but also speeds up the process of clinical trials. Faster trials can lead to quicker drug development times, benefiting the healthcare industry and patients alike. Moreover, the reliability of the data improves the trust in the trial results, ensuring that the findings are robust and can be used to make informed decisions about patient care.
The Strategic Role of CROs in Advancing DCTs
Navigating Regulatory Landscapes: CROs are instrumental in ensuring that DCTs comply with a complex array of regulatory requirements that vary by region and country. These regulations can include guidelines on patient privacy, data security, informed consent, and ethical standards for clinical trials. CROs' deep understanding of these legal frameworks helps to navigate through the bureaucratic hurdles that might otherwise impede the progress of a trial. They stay abreast of changes in legislation and implement these changes into the trial’s design and protocol, ensuring ongoing compliance.
Engaging Patients Effectively: Patient engagement is another critical area where CROs add significant value. In decentralized settings, where direct physical interactions are minimized, maintaining high levels of participant engagement becomes challenging yet essential. CROs develop and implement strategies to keep participants informed, involved, and motivated throughout the trial process. This can include:
Creating educational materials that help patients understand the trial's purpose and processes.
Implementing user-friendly digital tools for easy communication and data submission.
Offering support through various channels to address participants' concerns or issues promptly.
Managing Complex Data Logistics: Decentralized trials generate vast amounts of data from disparate sources such as wearable devices, mobile apps, and electronic health records. Managing this data—ensuring its integrity, security, and accessibility—is a complex task. CROs utilize advanced data management systems to collect, store, and analyze data securely and efficiently. They ensure that data handling procedures adhere to best practices in data privacy and cybersecurity, crucial in maintaining trust and credibility in the trial’s results.
Ensuring Ethical Conduct and Compliance: CROs are the stewards of ethical standards within clinical trials. They ensure that all aspects of the trial respect patient rights and welfare, from initial design to final reporting. This includes:
Ensuring informed consent is obtained transparently and includes all necessary information about the trial.
Monitoring for any adverse effects or issues during the trial and responding swiftly to mitigate risks.
Maintaining rigorous oversight to ensure that the trial does not deviate from ethical standards.
Focusing on Patient Safety and Data Accuracy: The ultimate goal of any clinical trial is to ensure patient safety and generate accurate, reliable data. CROs implement robust protocols and use cutting-edge technologies to monitor patient health and trial progress in real time, allowing for immediate intervention if safety concerns arise. They also establish stringent quality control measures to validate data accuracy, ensuring that the trial’s findings are valid and can withstand scrutiny from regulatory bodies and the scientific community.
Explore Courses for Clinical Research Career
Courses Available:
Conclusion
As we embrace the myriad innovations of Decentralized Clinical Trials in 2025, the potential for revolutionizing healthcare research is boundless. With CROs at the helm, ensuring meticulous execution and adherence to ever-evolving regulatory landscapes, the future is indeed promising. For those keen on navigating this thrilling domain, consider the comprehensive offerings at CCRPS. Dive into a world where clinical trials are not just a necessity but a seamless part of our digital lives, paving the way for medical breakthroughs that once seemed beyond our grasp. Embrace the change, become a part of the revolution, and let's redefine what's possible together in healthcare research!
Frequently Asked Questions (FAQs)
-
Decentralized Clinical Trials offer enhanced participant convenience, better retention rates, and access to a broader demographic, which can lead to more robust data and more generalizable research findings.
-
CROs provide specialized knowledge in regulatory standards, patient recruitment, data integration, and trial management, ensuring DCTs are conducted efficiently and within compliance frameworks.
-
Yes, by facilitating greater patient adherence, real-time data collection, and adaptive trial designs, DCTs can significantly improve the quality of research outcomes and speed up the drug development process