Good Documentation in Clinical Trials
Good documentation practices are pivotal in clinical trials to ensure data integrity, compliance, and reproducibility. These practices involve meticulous recording, timely entries, and careful validation of trial data. This accuracy ensures that clinical trials adhere to regulatory standards and scientific norms. Thus, robust documentation supports the validity and reliability of clinical research outcomes.
The medical record of the subject before, after, and during the clinical trial is a source document.
Characteristics of Source Document
It is useful to check if the person is suitable for a clinical trial or not. If you are a professional looking to improve your skills in handling clinical trials, then the Clinical Research Coordinator course and the Clinical Trials Assistant Training course are worth a look.
It records the evolution of the subject from consenting to the end of the randomized controlled trial that has been chosen.
It also helps track how much investigational product is used and returned by the subject, as well as how much is dispensed.
At any point of the treatment the source document is a complete medical record of the subject as a reference.
Finally it forms well built data. Then it is transcribed to CRF that translates it into a clinical study report.
The source document is consistent with the fundamental principles for the protection of the rights of subjects, their safety and well-being and is in accordance with the principles learned in the ICH-GCP course.
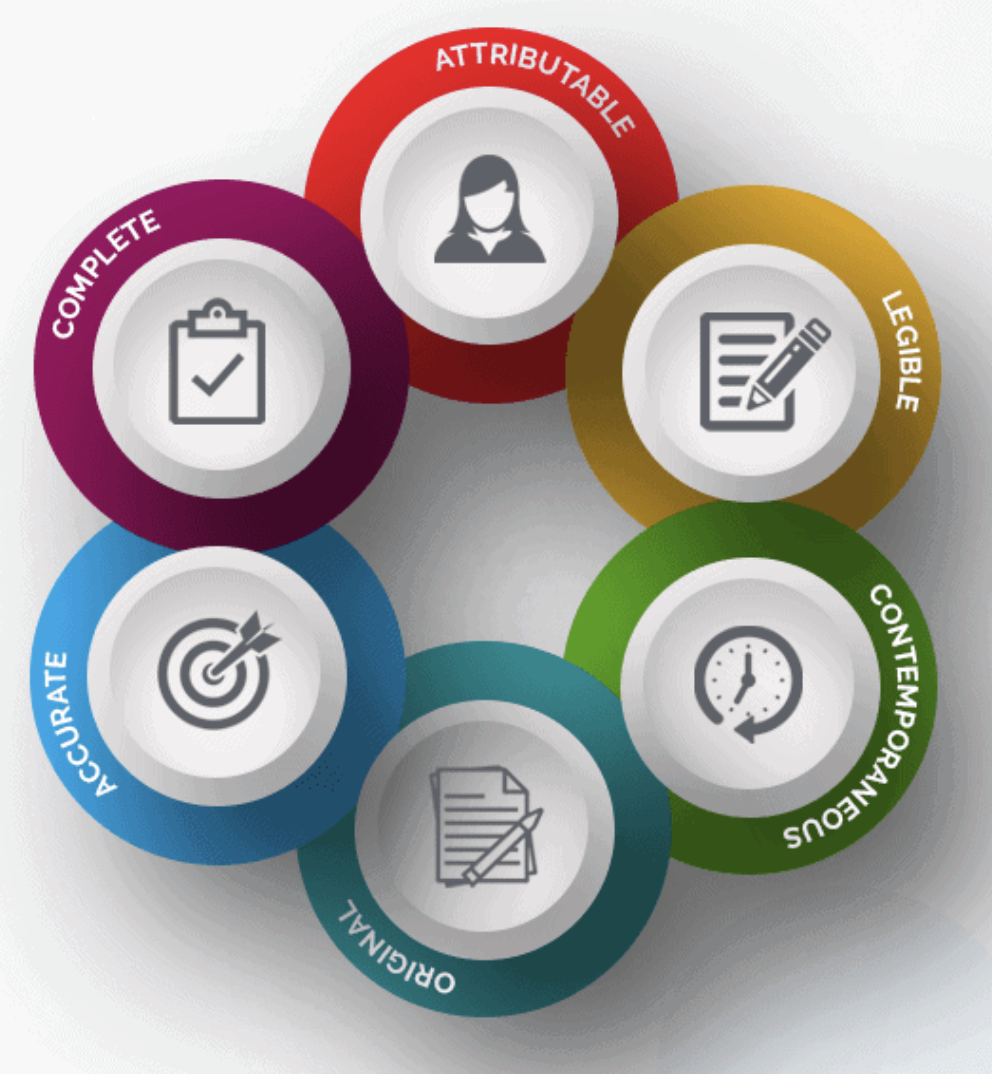
ALCOA-C
ALCOA-C incorporates all the primary elements of a source document as defined and documented by the U.S. FDA.
The reason why it got implemented in medical practices; is to ensure confidentiality, credibility, accuracy, and validation. ALCOA-C is the abbreviation of some crucial terms in clinical trials that are as follow:
Figure no. 1: ALCOA-C
Attributable
Legible
Contemporaneous
Original
Accurate
Enduring
Available and accessible
Complete
Consistent
Credible
Corroborated
For everyone involved in pharmacovigilance, the Pharmacovigilance Certification course can be seen as further training in how to guarantee drug safety and efficacy that adheres to these standards.
Types of a Source Document
There are two types of Source Documentation:
Electronic - An electronic record is defined as any form of text, graphics, data, audio, pictorial or other form of information in digital form that has been created, modified, maintained, archived, retrieved or distributed by a computer system. For more specialized training, consider the Advanced Clinical Research Project Manager Certification and the Medical Monitor Certification course.
Paper - Source Documents can take the form of pre-printed forms on which data has been written or it can be handwritten records. For the advanced certification as a Principal Investigator (PI) Physician, the topics related to the Levogen Drug Trial, Research Collaboration, and Ethical Responsibilities in Clinical Research seem to be key areas that those aiming to lead in this field should prioritize.
The most common type of Source Documentation (SD) is official medical documentation used in medical institutions regularly:
Medical History
Outpatient Medical Chart
Various Logs / Hospital Charts
For a comprehensive understanding of clinical trial documentation and monitoring, the CRA (Clinical Research Associate) course is highly recommended.
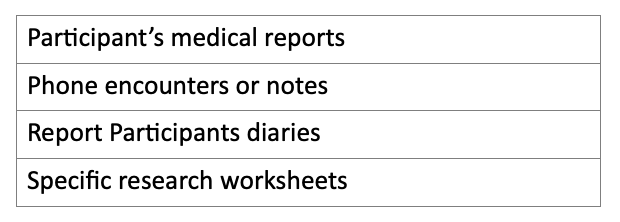
Table no. 1: examples of source document in clinical trials.
Examples of Source Document in Clinical Trial:
Participant’s medical reports
Phone encounters or notes
Report Participants diaries
Specific research worksheets
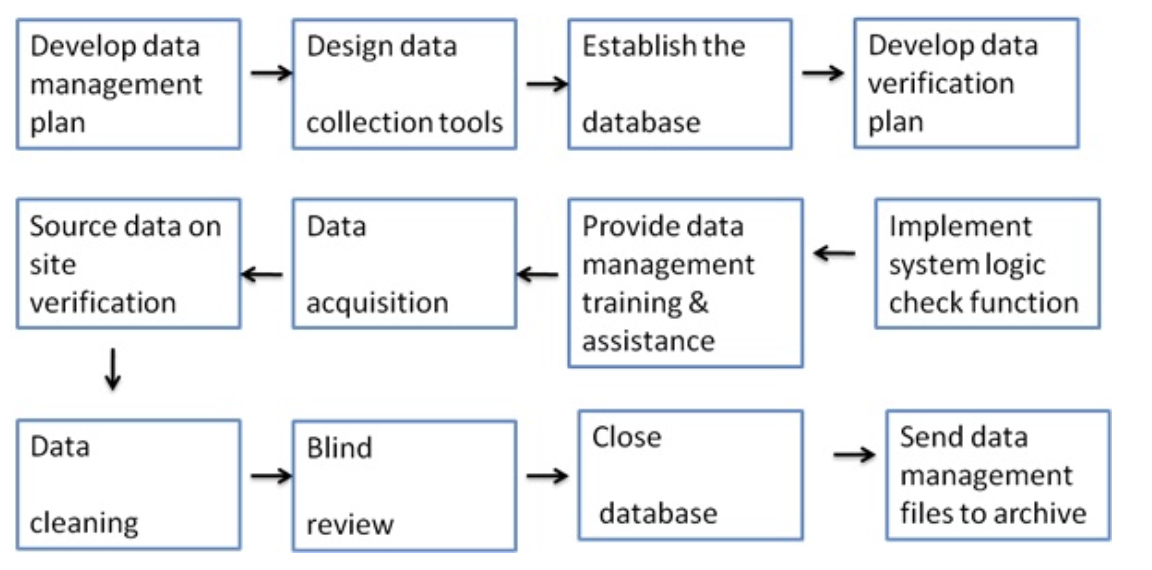
Figure no. 2: general flow chart for clinical data management.
Clinical Data Management
The collection, cleaning and management of subject’s data according to regulatory standards is called as clinical data management (CDM).
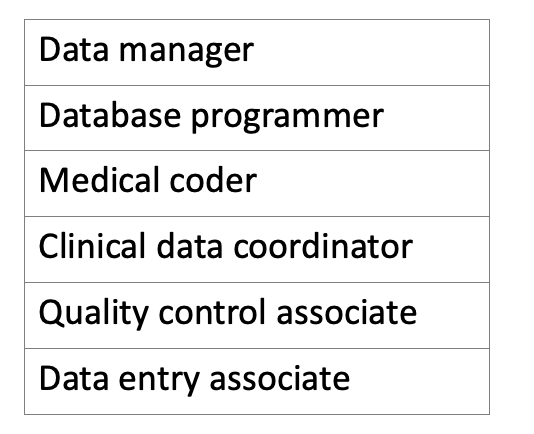
Table no. 2: tools for CDM.
Main Objectives of CDM:
To provide high-quality data.
To keep the number of errors and missing data as low as possible.
Try to get maximum data for analysis.
Table no. 3: minimum requirement for CDM team.
The information shall be in the electronic form signed by the signer in the CDM and must meet the requirements of the Code of Federal Regulation (CFR), 21 CFR Part 11. CRF uses the records in the electronic format that we have created, modified, stored, archived, retrieved, or transferred. ( binny et al., 2012)
Case Report Form:
CRF is the first step in the conversion of the protocol specific activities into the data that is being created. It should be brief, easy to use and understand. (binny et al., 2012)
Conclusion
In conclusion, good documentation in clinical trials underpins the foundation of effective clinical research. By adhering to ALCOA-C principles and ensuring comprehensive source documentation, researchers can safeguard subject safety and data integrity. The implementation of robust Clinical Data Management (CDM) systems further enhances data accuracy and reliability. For professionals aiming to deepen their understanding of these practices, the CCRPS offers specialized courses that align with current regulatory standards and industry best practices.
Explore Courses for Clinical Research Career
Courses Available:
References:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3121265/ - Good documentation practice in clinical research
https://conductscience.com/portfolio/alcoa-c/ - ALCOA-C
https://www.appliedclinicaltrialsonline.com/view/targeting-source-document-verification - Targeting Source Document Verification
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3326906/ - Data management in clinical research: An overview
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4170533/ - Basics of case report form designing in clinical research
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3857788/ - Remote Source Document Verification in Two National Clinical Trials Networks: A Pilot Study
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4386950/ - Impact of source data verification on data quality in clinical trials: an empirical post hoc analysis of three phase 3 randomized clinical trials
Frequently Asked Questions (FAQs)
-
Good documentation is crucial in clinical trials as it ensures the integrity, accuracy, and reliability of data collected. It supports compliance with regulatory requirements and helps protect participant safety and rights.
-
ALCOA-C enhances clinical trial documentation by emphasizing attributes like Attributability, Legibility, Contemporaneousness, Originality, and Accuracy, which are essential for ensuring the credibility and reliability of data in clinical trials.
-
The main types of source documents in clinical trials are electronic records and paper source documents. Each type plays a vital role in capturing comprehensive and accurate data throughout the trial process.
-
Clinical Data Management is vital as it involves the collection, cleaning, and management of trial data, ensuring high quality and minimizing errors. Effective CDM helps in making reliable decisions from clinical trials.
-
CCRPS provides a variety of certification courses aimed at enhancing skills in clinical trials management, including Clinical Research Coordinator, Clinical Trials Assistant Training, and Pharmacovigilance Certification, among others.