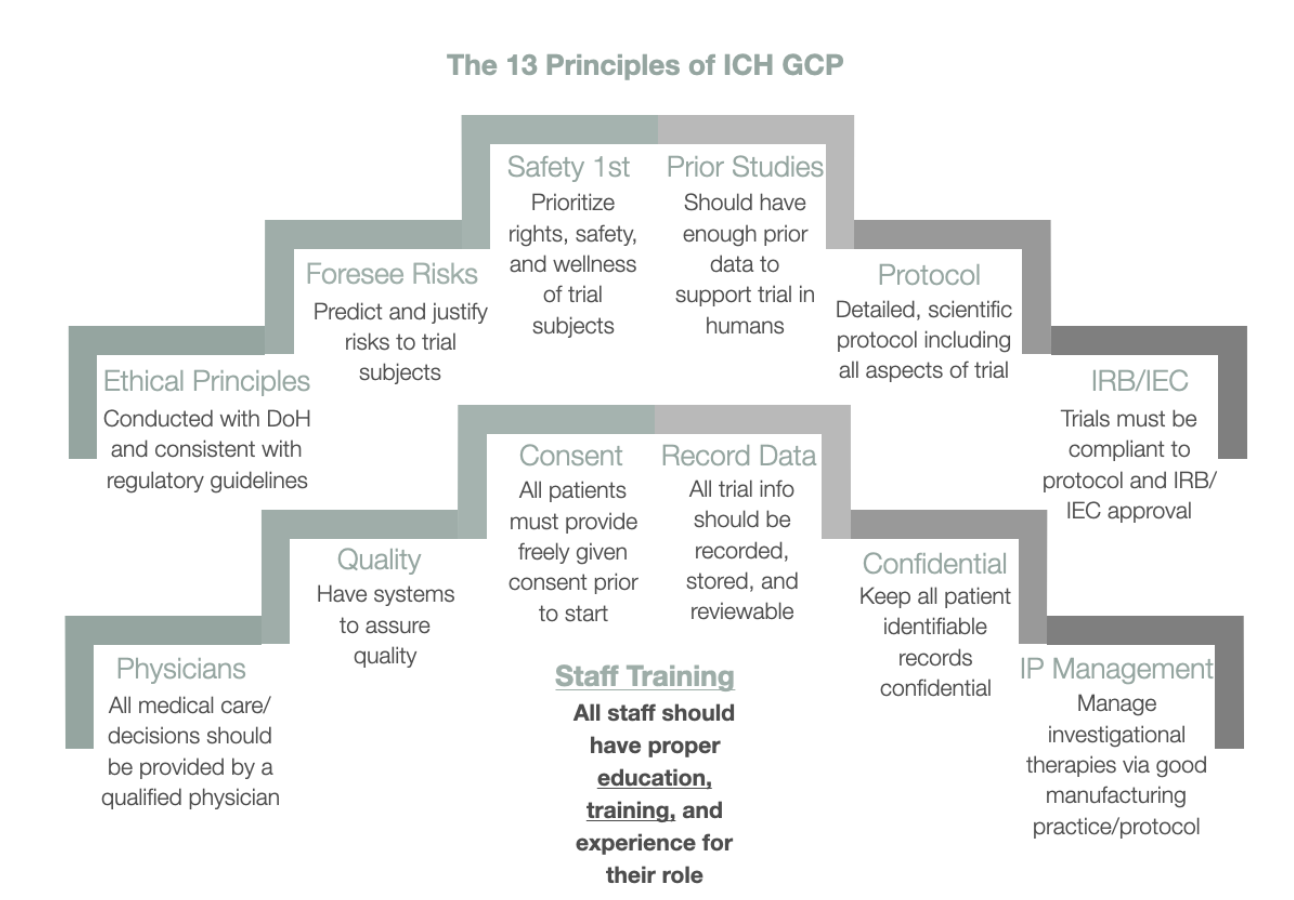
The Importance of ICH GCP
Why did the scientist go to the clinical trial? To spice up his routine with some trial and error! Jokes aside, diving into the realm of International Conference on Harmonisation - Good Clinical Practice (ICH GCP) is no laughing matter. It’s the backbone of clinical research that ensures the safety, rights, and well-being of participants while delivering credible data. So, grab your lab coat, and let’s unravel the critical significance of ICH GCP in the ever-evolving world of medicine.
Importance of ICH GCP
The Vital Role of ICH GCP (International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use - Good Clinical Practice) guidelines is multifaceted and critical to the foundation and execution of clinical trials worldwide. Here's a detailed breakdown of this passage to elucidate its importance:
International Standard
ICH GCP is recognized as the international benchmark for clinical trials. This harmonization is crucial because it ensures that drug regulatory authorities in different countries can trust the data generated from clinical trials. When countries adopt ICH GCP as their standard, it facilitates a more streamlined and unified approach to the clinical trial process, which is especially important in multi-national trials involving several countries.
Design and Protection of Human Subjects
The guidelines are meticulously designed to protect human subjects involved in clinical research. This protection is paramount as it assures potential and current participants that their safety, rights, and well-being are prioritized over all other aspects of the study. The emphasis on protection helps maintain public trust in the biomedical research process, which is essential for the continuation and success of clinical trials.
Ethical Practices
Ethical considerations are at the heart of ICH GCP guidelines. These include ensuring informed consent, respect for participants, and the right to withdraw from the study at any time without any repercussions. The ethical practices ensure that the trials are not only scientifically sound but also morally conducted, providing a clear ethical framework that guides all stakeholders in the conduct of clinical research.
Integrity of Biomedical Research
The integrity of biomedical research hinges on the reliability and validity of data collected during clinical trials. ICH GCP guidelines provide a comprehensive framework that includes standards for designing, conducting, monitoring, and reporting trials. This framework ensures that the data generated are accurate, reliable, and can be universally trusted. This integrity is crucial when new drugs are evaluated by regulatory bodies, which base their approvals on the data from these trials.
Simplification of the Global Submission Process
By establishing a common set of standards, ICH GCP simplifies the global submission process for new drugs. Pharmaceutical companies benefit from this as they do not need to redo or adjust clinical trials to meet different national standards, which can be both time-consuming and costly. This not only speeds up the drug approval process but also helps new therapies reach the market faster, benefiting patients worldwide.
Facilitating Cross-Border Acceptance
One of the significant advantages of ICH GCP guidelines is the facilitation of cross-border acceptance of clinical data. When countries recognize and accept data from trials conducted in other nations that follow ICH GCP, it eliminates redundancies and accelerates the review process. This global acceptance is essential for the rapid development and distribution of vital medications, particularly in areas like global health emergencies where time is of the essence.
Fig 1: Good clinical practice and ICH
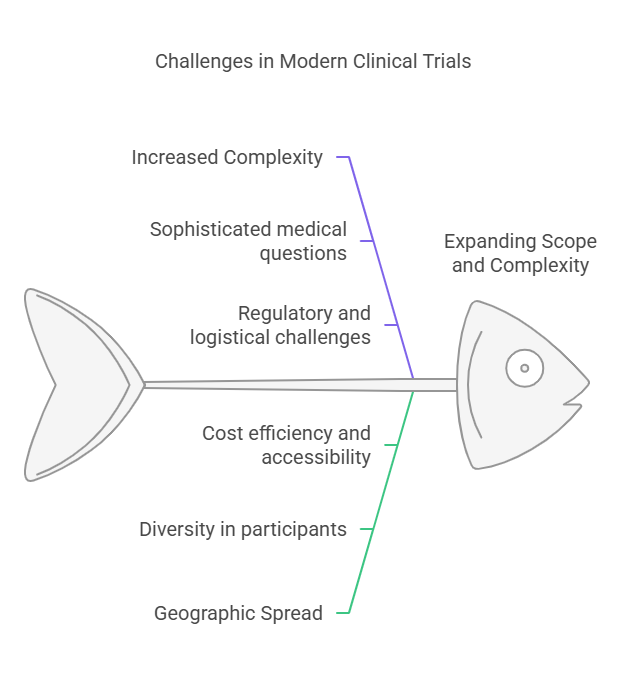
Expanding Scope and Complexity
The passage "Expanding Scope and Complexity" addresses how clinical trials have grown not only more complex but also more widespread geographically over recent decades. This expansion is crucial to understanding the current landscape of clinical research, especially in the context of globalization and the rapid development of new medical treatments and technologies. Here’s a deeper look into each element of this expansion:
Increased Complexity
Clinical trials today deal with more sophisticated medical questions and technologies than ever before, including genetic therapies, complex biologics, and personalized medicine. The complexity also comes from the regulatory requirements and logistical arrangements needed to manage multi-center trials that span different regulatory environments. Each of these factors adds layers of complexity in trial design, data management, and compliance.
Geographic Spread
The geographic expansion of clinical trials refers to conducting studies across a global landscape—from major cities in developed countries to remote areas in the developing world. This global reach is essential for several reasons:
Diversity in Participants: It allows for a diverse population sample in trials, which is critical for understanding how drugs perform across different genetic backgrounds and environments.
Accessibility: Expanding into less developed areas can improve access to new therapies in regions where healthcare might be less advanced.
Cost Efficiency: Sometimes, trials are conducted in countries where the cost of trial execution is lower, which can make large scale studies more feasible.
Role of ICH GCP
In managing both the complexity and the spread of trials, ICH GCP guidelines provide a unified framework that ensures consistency and integrity in how clinical trials are conducted globally. They detail how to address logistical, ethical, and regulatory challenges across different jurisdictions. By following these guidelines, trials can ensure that their data will be accepted by major regulatory bodies worldwide, thus supporting the global marketing of new therapies.
Training for Excellence
The passage "Training for Excellence" emphasizes the necessity and benefits of comprehensive training in Good Clinical Practice (GCP) for all individuals involved in clinical research. Here’s what this entails:
Mandatory Training
Training in ICH GCP is not merely beneficial; it is a regulatory requirement for everyone involved in the conduct of clinical trials. This requirement ensures that all personnel—from the principal investigators who lead the trial to the clinical research coordinators who manage day-to-day operations—are proficient in their roles and understand the regulatory standards they must meet.
Scope of Training
GCP training covers a broad range of topics essential for the ethical and effective management of clinical trials, including:
Ethical Conduct: Ensuring the protection of human subjects and adherence to informed consent procedures.
Trial Management: Understanding how to design, conduct, monitor, and report on clinical trials to ensure data integrity and compliance with regulatory standards.
Handling Emergencies: Preparing staff to manage unexpected situations or complications during the trial process.
Benefits of Training
Proper GCP training ensures that clinical trials are conducted efficiently and ethically, maintaining high standards of data quality and participant safety. Well-trained personnel are more likely to:
Prevent Violations: Avoid common pitfalls and regulatory non-compliance that can lead to trial delays or cancellations.
Enhance Data Integrity: Produce reliable, robust data that can withstand scrutiny by regulatory authorities.
Uphold Participant Dignity and Safety: Ensure that the rights and well-being of trial participants are always the primary concern.
Explore Courses for Clinical Research Career
Courses Available:
Conclusion
The ICH GCP guidelines are not just a set of rules but a manifesto for ethical clinical research. As we continue to explore new frontiers in medicine, adhering to these principles is paramount. Remember, behind every clinical trial and data point are human lives trusting us with their well-being. For those looking to deepen their expertise, consider the Certified Clinical Research Professional (CCRPS) certification, a beacon for those aspiring to excel in clinical research.
Frequently Asked Questions (FAQs)
-
ICH GCP is a set of globally recognized ethical and quality standards for conducting clinical trials, ensuring the protection and safety of participants.
-
It standardizes trial practices across borders, enhancing global drug development and approval processes.
-
It ensures that their rights, safety, and well-being are prioritized throughout the trial process.
-
Training focuses on ethical conduct, risk management, protocol adherence, and data integrity.
-
Yes, they are periodically updated to reflect new ethical concerns, scientific advancements, and technological developments.