How to Become Certified in Good Clinical Practice
Good Clinical Practice (GCP) is a critical certification for professionals in the field of clinical research. It ensures that individuals involved in clinical trials maintain high standards of patient safety, data integrity, and ethical conduct. Becoming GCP certified can boost your career and provide a competitive advantage in the clinical research industry. In this blog, we will provide a comprehensive, step-by-step guide on how to obtain GCP certification, along with recommended courses, exam preparation tips, costs, and the career benefits of certification.
Related Blog: GCP Certification: What You Need to Know
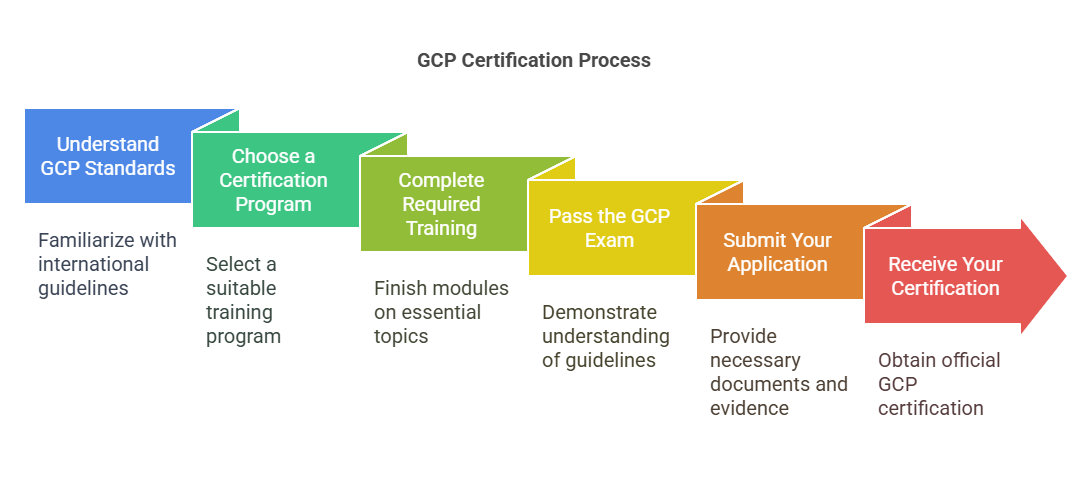
Step-by-Step Guide to Obtaining GCP Certification
Obtaining GCP certification involves a series of steps that ensure you have the necessary knowledge and skills to work in clinical trials. Here’s a breakdown of the process:
Understand the GCP Standards:
Before seeking certification, familiarize yourself with the basics of GCP. This includes the international guidelines established by regulatory bodies such as the FDA, EMA, and ICH. GCP covers everything from patient safety, ethical guidelines, to data management in clinical trials.
Choose a Certification Program:
There are various training programs available, both online and in-person. It is crucial to choose a program that offers in-depth knowledge of GCP and aligns with your career goals.
Complete the Required Training:
Training programs typically consist of modules that cover essential topics such as patient safety, ethical guidelines, adverse event reporting, and more. Many programs also provide access to real-world scenarios and case studies.
Pass the GCP Exam:
After completing your training, you will need to pass a GCP exam to demonstrate your understanding of the guidelines. The exam will test your knowledge on various aspects of clinical trials, including ethical conduct, patient rights, and safety protocols.
Submit Your Application:
Once you’ve completed the training and passed the exam, you will need to submit your certification application. Some organizations may require additional documents or evidence of clinical trial experience.
Receive Your Certification:
After your application is reviewed, you will receive your GCP certification. It is often valid for a certain number of years, after which you may need to renew it.
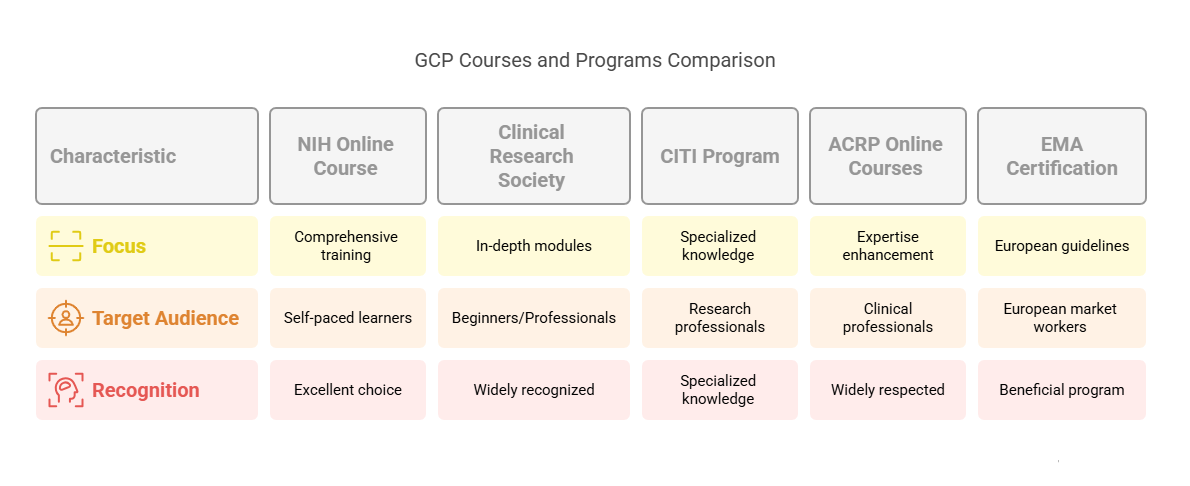
Recommended GCP Courses and Programs
Choosing the right course or program is essential for obtaining proper training. Below are some reputable GCP courses and programs that will equip you with the knowledge and skills needed to succeed:
Online GCP Certification Course by The National Institutes of Health (NIH):
This free, online course offers comprehensive training in GCP, covering all major aspects of clinical trials and patient safety. It’s an excellent choice for those looking to learn at their own pace.GCP Certification by the Clinical Research Society:
This certification program is widely recognized in the industry. It offers in-depth modules on GCP standards, trial management, and regulatory compliance. It's ideal for both beginners and professionals.CITI Program - GCP for Clinical Research:
The Collaborative Institutional Training Initiative (CITI) offers a series of courses focused on GCP for clinical trials. These are ideal for professionals looking for specialized knowledge in various research areas.Online Courses by ACRP (Association of Clinical Research Professionals):
ACRP offers online GCP training programs that are widely respected in the clinical research community. The courses are designed for professionals seeking to enhance their expertise in clinical trials.GCP Certification by the European Medicines Agency (EMA):
The EMA offers training programs focused on European GCP guidelines. This program is especially beneficial for those working with European clinical trials or looking to work in European markets.
These courses provide a solid foundation in GCP and are offered by reputable organizations recognized within the clinical research community.
Related Blog: How Good Clinical Practice Improves Patient Safety
Duration and Cost of Certification
The duration and cost of obtaining GCP certification vary depending on the program you choose. Here are some general guidelines:
Duration:
Online Courses: Most online GCP courses are self-paced, allowing you to complete them in your own time. On average, the training can take anywhere from 2 to 8 hours to complete, depending on the depth of the course.
In-Person Courses: In-person GCP certification programs typically span 1-2 days. Some may offer follow-up training or workshops, which can extend the duration.
Cost:
Free Courses: Some institutions, such as the NIH, offer free GCP certification programs. These are great for individuals starting their career in clinical trials or those seeking basic certification.
Paid Courses: Paid courses typically range from $100 to $500. More specialized programs or certifications from professional bodies like ACRP or CITI can cost upwards of $1,000.
Additional Fees: In some cases, there might be an additional fee for the exam or certification issuance. Make sure to verify these details before enrolling in a program.
Choosing a course that fits your budget while offering high-quality content is essential for effective learning.
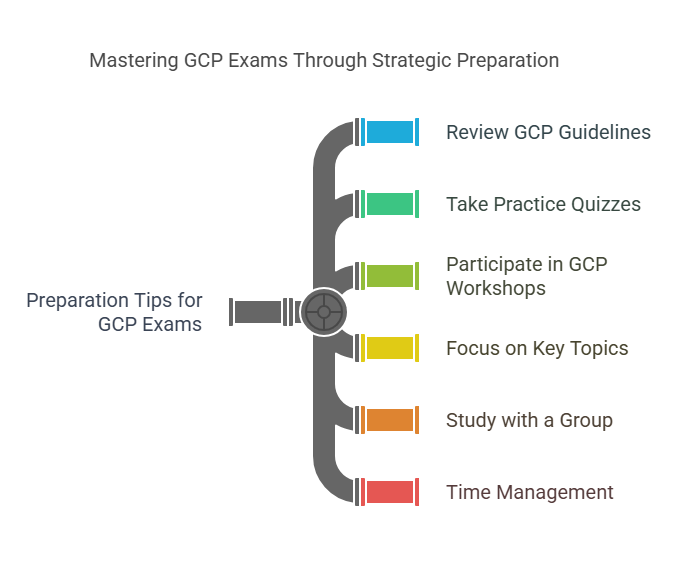
Preparation Tips for GCP Exams
The GCP exam tests your knowledge of clinical trial processes, patient safety, regulatory requirements, and ethical considerations. Here are some tips to help you prepare effectively:
Review GCP Guidelines Thoroughly:
Study the guidelines issued by ICH, EMA, and FDA. Familiarize yourself with common terms, ethical considerations, and regulatory requirements.Take Practice Quizzes:
Many certification programs provide practice quizzes or mock exams. These quizzes will help you assess your knowledge and identify areas where you need further study.Participate in GCP Workshops:
Workshops and webinars provide opportunities to discuss real-world clinical trial scenarios and the application of GCP principles. They can help solidify your understanding.Focus on Key Topics:
Concentrate on areas like patient safety, informed consent, adverse event reporting, and ethical guidelines, as these are often heavily featured in the exam.Study with a Group:
If possible, join a study group. Discussing key concepts with others can help reinforce your understanding and clarify any doubts.Time Management:
Set aside regular study sessions and pace yourself. Don’t rush through the material, as understanding the concepts deeply is more beneficial than just memorizing them.
Career Benefits of Becoming GCP Certified
Obtaining GCP certification comes with a wide range of career benefits, especially in the clinical research field. Here's how becoming certified can enhance your professional life:
Improved Job Prospects:
GCP certification is recognized globally, and having it on your resume shows that you are committed to high standards in clinical research. Many employers require GCP certification for clinical trial professionals, making it a must-have credential.Higher Earning Potential:
Certified clinical research professionals often earn more than their non-certified counterparts. GCP certification can lead to salary increases, promotions, and higher-paying job opportunities.Increased Job Security:
In a competitive industry, GCP certification helps ensure that your skills are up-to-date with the latest industry standards. This can increase job stability and open up opportunities for advancement.Career Flexibility:
GCP certification allows you to work in a wide variety of settings, from pharmaceutical companies to research institutions and regulatory bodies. It gives you the flexibility to explore different areas of clinical trials and medical research.Professional Credibility:
As a GCP-certified professional, you will be seen as a reliable and knowledgeable member of the clinical research community. This credibility can lead to more responsibilities, leadership roles, and professional recognition.
10 Lesser-Known Facts About GCP
GCP is not just for clinical researchers; it is also essential for physicians, nurses, and other healthcare professionals involved in clinical trials. (Source)
GCP was originally developed by the International Conference on Harmonisation (ICH) to harmonize regulatory requirements for clinical trials across different countries. (Source)
GCP is a requirement for receiving funding from regulatory authorities like the FDA and EMA for clinical trials. (Source)
Many hospitals and healthcare organizations require their staff to be GCP certified before they can participate in or manage clinical trials. (Source)
There are different GCP standards for different regions, such as ICH-GCP (international), FDA-GCP (USA), and EMA-GCP (Europe), but they share many common principles.
GCP includes rules for maintaining patient privacy and ensuring that personal health information is kept confidential throughout the trial process.
Renewal of GCP certification is required every 2-3 years, depending on the certifying body, to ensure that professionals remain updated on new guidelines and regulations.
GCP certification is applicable to more than just pharmaceutical studies; it also applies to medical device trials, clinical investigations, and even some healthcare technology assessments.
Some GCP courses offer CEUs (Continuing Education Units), which may be required to maintain professional licenses for healthcare professionals.
GCP training is often part of larger certification programs for clinical research associates (CRAs), clinical research coordinators (CRCs), and clinical data managers (CDMs).
Explore Courses for Clinical Research Career
Courses Available:
Conclusion
Becoming certified in Good Clinical Practice (GCP) is a valuable step for anyone looking to advance their career in clinical research. The process involves completing a training program, passing an exam, and demonstrating your understanding of ethical guidelines, patient safety, and regulatory requirements. By following the recommended training courses, managing your preparation effectively, and leveraging the career benefits, you can enhance your professional credentials and open doors to new opportunities.
In conclusion, obtaining your GCP certification through programs supported by CCRPS can provide you with the skills, knowledge, and credibility necessary to excel in the field of clinical trials and patient safety.
-
GCP certification is an internationally recognized credential that confirms an individual’s knowledge of clinical trial guidelines, patient safety protocols, and ethical standards.
-
To obtain GCP certification, you must complete a GCP training program, pass the associated exam, and submit an application for certification.
-
The cost of GCP certification varies, with free courses available, and paid programs typically ranging from $100 to $500. Some professional certifications may cost more.
-
The duration of GCP certification programs ranges from 2 hours to a few days, depending on whether you choose an online or in-person course.
-
GCP certification enhances job prospects, increases earning potential, improves job security, and provides career flexibility in the clinical research field.