(CRC)
Clinical Research Coordinator Certification
Guide To Becoming A Clinical Research Coordinator
What is a Clinical Research Coordinator
Clinical research coordinators play a crucial role in administering clinical trials, overseeing various aspects such as data collection, participant engagement, and adherence to trial standards. As of 2024, their responsibilities have expanded to include ensuring smooth trial operations and effective communication with subjects. Here's a revised version of your content:
Clinical research coordinators are pivotal in the administration of clinical trials. Their primary responsibilities typically involve administering questionnaires, informing participants about study objectives, collecting data, and managing all trial procedures. In 2024, their roles have evolved to encompass strict adherence to established trial standards and active involvement in participant recruitment.
Effective engagement with subjects is paramount for clinical research coordinators, necessitating strong communicative and interpersonal skills.
Responsibilities
Clinical research coordinators shoulder a multitude of responsibilities, all critical to the success of their endeavors:
Maintaining meticulous records of all studies in compliance with guidelines.
Adhering rigorously to ethical standards governing research.
Ensuring compliance with regulatory standards.
Administering questionnaires and other study protocols.
Managing the research budget efficiently.
Overseeing the smooth execution of trials.
Engaging with participants to address concerns and gather insights.
Ensuring functionality and availability of necessary equipment and supplies.
Participating actively in participant recruitment efforts.
Collaborating with laboratories to share findings.
Requirements
Qualifications for clinical research coordinators vary depending on location and employer. However, common requirements as of 2024 include:
An associate nursing degree or related field.
A minimum of two years' experience in the healthcare industry.
An analytical mindset and keen attention to detail.
Exceptional interpersonal skills for effective participant engagement.
Willingness to pursue continuous learning opportunities independently.
Strong organizational abilities.
Excellent verbal and written communication skills.
In a dynamic field like clinical research, staying abreast of advancements and regulations is essential for success.
This revision integrates the existing content with updates reflecting the current landscape in 2024, emphasizing the evolving nature of clinical research coordination.
Roles And Duties Of Clinical Trial Coordinator
In 2024, the part of a clinical investigate facilitator includes overseeing clinical inquire about at investigate destinations in understanding with convention, ICH-GCP rules, and other administrative necessities. Understanding the errands of a clinical investigate facilitator requires understanding into the timeline of a inquire about location, which regularly unfurls in three stages:
Some time recently Beginning the Clinical Trial:
During this arrange, consider organizers assemble and total surveys from supports and different Contract Inquire about Organizations (CROs). Clinical inquire about facilitators at that point collect information from the foremost examiner and transfer it back to partners. Supports select areas based on achievability survey reactions and conduct pre-site evaluation visits to finalize taking an interest sites.
Sites conducting investigate must hold clinical trial facilitator certification to continue with agent gatherings, regularly held at universal or national levels. Some time recently commencing the trial, clinical inquire about facilitators are possessed with submitting different reports to the morals committee, counting subject journals, investigators' CVs, clinical investigate assentions, convention signature pages, certification, indemnification letters, protections certificates, clear Case Report Shapes (CRFs), and ponder logs.
Conduct Amid the Clinical Trial:
Clinical investigate facilitators must have a exhaustive understanding of the think about convention, counting avoidance and consideration criteria. They get educated assent from subjects, as spoken to by Central Examiners, and collect pre-medical records. Facilitators oversee planned visits agreeing to the examination convention, guaranteeing compliance with prohibition and consideration criteria some time recently selecting qualified subjects. Taking after each visit, facilitators compile information into case reports, keeping up overhauled records all through the trial.
Coordinators are capable for overseeing ponder medicate responsibility, utilizing Intelligently Web Reaction Frameworks (IWRS) and Intuitively Voice Reaction Frameworks (IVRS) to record subject visits. Legitimate capacity and dealing with of investigational items, counting temperature observing, are pivotal. Facilitators too collect essential information on unfavorable occasions, observing lab reports and securing Vital Agent signatures.
After Completing the Clinical Trial:
Upon trial completion, facilitators audit and upgrade all reports some time recently closure, guaranteeing precision and completeness. Clinical Inquire about Partners (CRAs) confirm materials on the trial's last day. Facilitators help in chronicling records at the location, keeping up records for 15-20 years.
In outline, clinical investigate facilitators play a essential part in managing clinical trials at the location level, serving as a crucial connect between morals committees, examiner destinations, and supports. Their fastidious administration guarantees compliance, information judgment, and the fruitful execution of clinical investigate endeavors.
Education Requirements Of A Clinical Research Coordinator
To set out on the travel of getting to be a Clinical Investigate Facilitator in 2024, one must take after a organized way. Here are the steps you require to take:
Step 1: Tall School Graduation
Completion of tall school lays the establishment for your scholastic travel. Center on subjects like material science, chemistry, science, arithmetic, measurements, and communication to construct a solid base for your future studies.
Step 2: Bachelor's Degree
Seek out colleges and colleges advertising bachelor's degrees in wellbeing sciences. These programs prepare understudies with the vital devices and techniques for research facility work, definition of solutions, and conducting clinical trials and thinks about. Whether through on-campus or online courses, guarantee the educational programs covers both authoritative and logical angles basic for a clinical investigate coordinator.
Step 3: Work Experience
Gain down to earth encounter by volunteering at clinical trials and securing entry-level positions in teach or investigate research facilities. Investing a year or two in this capacity will give profitable hands-on encounter pivotal for certification and employment.
Step 4: Online Graduate Certificate
Consider seeking after an online graduate certificate in clinical investigate organization. These programs regularly span 18 credit hours, displaying devotion and improving your career prospects. Courses center on administrative and communication aptitudes, inquire about plan, location administration, information administration, measurements, member security contemplations, and more. These credits can regularly be exchanged to a master's program in the same institution.
Step 5: Master's Degree (Online or On-site)
Advance your information and career prospects by getting a master's degree, either online or through conventional on-site courses. Master's programs dive more profound into essential administrative issues and clinical inquire about checking, giving comprehensive planning for certification.
Step 6: Get Certification
To hone as a certified Clinical Inquire about Facilitator, you must pass an exam and get certification. Organizations like CCRPS offer globally recognized certification programs. Upon certification, you'll be well-equipped to set out on a satisfying career in clinical inquire about coordination.
By perseveringly taking after these steps, trying people can clear the way towards getting to be capable Clinical Inquire about Facilitators in the energetic field of restorative inquire about.
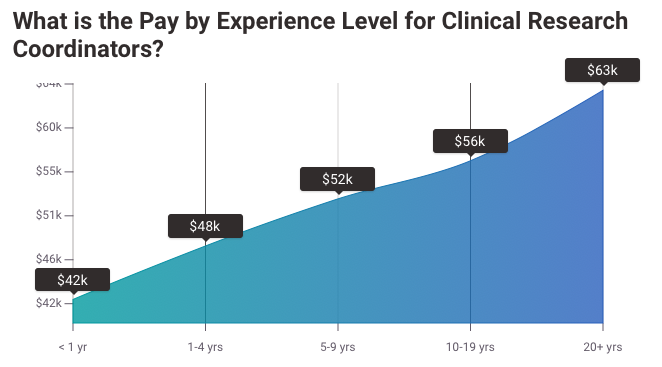
Salary Of A Clinical Research Coordinator
Salary of a Clinical Research Coordinator
How Much is a Clinical Research Coordinator’s Salary?
As of Walk 5, 2024, the normal yearly pay for a Inquire about Facilitator in the Joined together States is $80,570. This deciphers to around $38.74 an hour, $1,549 per week, or $6,714 per month.
While pay rates for Investigate Facilitators can shift essentially, with a few gaining as much as $112,500 yearly and others as moo as $21,500, the larger part drop inside the extend of $61,000 (25th percentile) to $99,500 (75th percentile). Beat workers, speaking to the 90th percentile, can make up to $110,000 per year.
The impressive variety in compensations, up to $38,500, recommends various openings for progression and expanded pay based on components such as expertise level, area, and a long time of experience.
Based on later work posting action, the Investigate Facilitator work advertise in the Joined together States is not especially dynamic. Be that as it may, certain cities offer higher-than-average pay rates for Inquire about Facilitators. Among the beat 10 most noteworthy paying cities are Berkeley, CA; Modern York City, NY; and Renton, WA. These cities offer normal pay rates surpassing the national normal, with Berkeley, CA, driving with an normal compensation surpassing $97,543.
While migrating to these cities may show openings for financial progression, it's basic to consider components such as the fetched of living and the generally little variety in normal compensations among the best cities. This recommends restricted potential for critical wage increments based exclusively on area.
Free Online Clinical Research Coordinator Training
Free courses for CRC training are available is specific subjects
You can search for free courses on the following subjects to get training that a CRC may benefit from:
Medical Ethics Course: - In this course, the professors introduce important values, which include autonomy, non-maleficence, dignity, justice, and honesty. The students consider how to develop a framework for creating ethical decisions that were informed by laws and values. You will discuss ethical issues, like favorable cost-benefit ratio, participant selection, and confidentiality. It is one of the best clinical research coordinator certifications online courses to help you to crack the exams.
Clinical Research Principles Course: - This clinical research coordinator certifications online course will provide an overview of the process of clinical research, development, and the history of it. Here the students can quickly learn the management skill, which includes the practice guidelines of clinics. You will also learn about the roles of research team members and development phases of the clinical trials.
Medical Terminology Course: - This clinical research coordinator certification online courses cover up the standard medical terminology which uses up in the clinical research field. This knowledge will help enhance your effectiveness at managing the data and quality control.
Health Information And The Law Course: - In this clinical research coordinator certifications online course, the students need to understand the overview of guidelines and the regulations which protect the human subjects and ensures research integrity. T You will learn about the obligations of the regulatory bodies. Here, students will even discuss the types of violations constituted by scientific misconduct and consider their consequences.
Introduction To The Health Records Courses: - This clinical research coordinator certifications online explore the confidentially and its purpose. This course will consist of how to use medical records for planning, the caring of the patient, and how to use laws for these records.
Study Of Financing Course: - This clinical research coordinator certifications online will provide you an overview of funding management of the study. Here students get to learn how to submit the proposals and how to create them. This course compiles the financial regulations, which consider the indirect and the direct cost.
Medical Device History: - In this clinical research coordinator certifications online course, it will provide you to explore the current trends which are affecting the research, which also explores the history of devices in medical. Students will be provided with a lot of case studies that can be considered from a business, medical, ethical, and also legal perspective.
These are a few free clinical research coordinator certifications online courses that you can go for preparing for your clinical research certifications. These courses can help you a lot in every perspective of exams, but the best option is to take a course that can provide you with in-depth accredited training. Overall, getting accredited certification from a trusted body such as CCRPS is the best option in showing employers your competency for coordinator roles.
CRC Course Syllabus
Introduction to CRC
Accreditation Council For Clinical Research & Education for CCRPS
Duties and Responsibilities of Clinical Research Coordinators
Employment Advancement for Clinical Research Coordinators
Process Map of A Sponsored Clinical Trial Study
Orientation Manual for Clinical Research Coordinator
Protocols and Guidelines
SOPs and MOPs
SOP Template
MOP Outline
MOP Example
Clinical Research Coordinator Toolkit
Routine Site Visit Report
Adverse Event Tracking Log
Chart Audit Tool
Regulatory File Review Tool
Monitoring Log
ICH GCP
An Introduction to Clinical Research
An Overview of ICH GCP
Code of Federal Regulations
CFR 21 Part 11
Sponsor/CRO Responsibilities
ICH GCP E6 Sections 2-4 Principles, IRB, & Investigator Roles
Reporting Responsibilities of the Investigators
Ethics of Research Involving Children
Ethics of Research Involving Pregnant Women and Fetuses
Ethics of Research Involving Prisoners
ICH GCP E6 and E2A - Adverse Events
ICH GCP 5.5 Trial Management – Data Handling and Record Retention
a) Common Terminology Used In Clinical Research
b) Commonly Used Abbreviations and Terms in Clinical Research
ICH GCP Quiz
Advanced Clinical Trials Foundations
Designs of Clinical Trials
Stakeholders in Clinical Research and Their Relationships
Site and Investigator Selection
Site Initiation Visit (SIV)
Site Qualification Visit
Routine Monitoring Visit
Site Close Out Visit
Source Documents and Informed Consent Forms
Quality Monitoring Quiz Modules 1-15
Inclusion Exclusion Criteria in Clinical Research
Interactive Voice Response System - IVRS
Protocol Deviations and Violations
Institutional Review Board
Quality Control in Clinical Research
Blinding in Clinical Trials
Communication between Blinded and Unblinded Staff
Investigational Product Accountability in Clinical Trials
Quality Monitoring Quiz
Adverse Drug Reactions
Basics of Adverse Event Monitoring
Adverse Event Reporting
Safety Reporting Requirements for Sponsor Investigators of An IND
IND and NDA Process
Do’s and Don’ts of a Case Report Form Design
Compliance and Regulations
Regulatory Documents in Clinical Research
Regulatory Affairs
Essential Regulatory Documents Guidance and Binder Tabs (Part 1)
Essential Regulatory Documents Guidance and Binder Tabs (Part 2)
Financial Disclosure- Duties and Strategies for Clinical Studies
Financial Disclosures and Conflicts of Interest in Clinical Research
FDA Form 1572 - Part 2
Delegation of Authority Log – DOAL
Investigators Brochures
Protocol Continuing
IND Application
Trial Master File Reference Guide
Regulatory Training Quiz (20 Questions)
Audit and Inspections
FDA Warning Letter
Site FDA Audit Inspection Checklist
How to Survive Through an FDA Inspection
Do and Don’ts during an FDA Inspection
Audits and Inspection Quiz
Subject Recruitment and Retention
Compliance Requirements in Clinical Trials
Subject Recruitment and Retention (Part 2)
Increasing Subject Compliance in Clinical Trials
Ethical Consideration Associated with Investigator Payment and Patient Recruitment
Advertisement Aid in Subject Recruitment and Retention
Misconduct and Fraud
Misconduct in Research – Detecting Falsification
Statistics and Data Management of Clinical Trials
Data Management In Clinical Research
Good Clinical Data Management Protocol
Financial Management of Clinical Trials
Financial Management Fundamentals
Developing A Trial Budget
Budget Worksheet
Final Examination
Competency Exam (52 Questions)
Are you looking for a comprehensive and reliable training program for clinical research coordinator certification?
CCRPS Clinical Research Coordinator Training provides the most advanced, yet easy-to-follow coverage of GCP guidelines. Our program exceeds expectations with checklists, images, and examples that help students apply concepts learned. Upon completion of our program, students are able to pass certification exams with flying colors and are trusted by employers all over the world. Make the smart choice and choose CCRPS for CRC training.
Clinical Research Training For Nurses: A Guide to Becoming a Clinical Research Nurse
CLINICAL RESEARCH TRAINING FOR NURSES
Guide to Becoming a Clinical Research Nurse
What is Clinical Nursing Research?
Nurses are known for providing direct care for patients. However, nurses may take up roles that are completely new to them within the world of clinical research. These roles include clinical research coordinator, educator and manager. They can also take up less traditional role like regulatory specialist, study monitor and IRB (institutional board review) admin.
Regulatory specialist: their activities relate mainly with preparing regulatory documents and communicating with regulatory bodies. Nurses can work as a regulatory affairs specialist, a regulatory operation coordinator, or a regulatory coordinator. They can work within government agencies, pharmaceutical companies, academic medical centers.
Study monitor: they monitor clinical research practices and make sure that it complies with necessary research protocols and regulations. They tend work at government agencies, biotechnology companies, pharmaceutical companies, contract research organizations, device manufacturers etc. Aspiring study monitors can enhance their qualifications with a Pharmacovigilance Certification.
Institutional Review Board (IRB) administrator: they are the professionals in charge of overseeing, administrating, implementing and managing IRB activities, like policies and procedures that relates to protecting human welfare. They can work at all IRBs: local, commercial or central IRB.
Nurses that have developed interest in the field of clinical research can join professional organizations. This provides them with the opportunity to network and continue their education through mediums like conferences, webinars, discussion groups, publications and online resources. These avenues serve as part of their clinical research training.
Certification is often a parameter used to measure professional expertise. This is based on criterion that reflects skill, knowledge, educational preparation, ability, and competence that are developed from experience in that area of specialization. Nurses that developed an interest in clinical research and have taken a clinical research training program have an opportunity to be certified through the:
Society for Clinical Research Professionals, Inc. (Certified Clinical Research Professionals)
Association for Clinical Research Professionals (Certified Clinical Research Associate or Certified Clinical Research Coordinator)
This field of clinical research gives nurses a chance, an opportunity to advance themselves professionally in a field that might not have been explored by them before. The benefits of having a registered nurse cover letter are insurmountable. This also provides a career path that can show family members the benefits of working in the medical field.
Nurses that have gone through the clinical research for nurses, otherwise called research nurse can carry out research on the various aspects of the human health, such as illness, pharmaceutical and health care methods and treatment plans. The main aim of this research is to improve the quality of health care service delivery. Helping patients and their family in a healthcare facility also brings a level of joy that is hard to find in many other career paths.
Roles of Research Nurses
They are responsible for designing and implementing research studies.
They observe procedures for treatment, collect and analyze data.
They report their research results to appropriate quarters.
They write articles and report their research findings in nursing or medical professional publications and journals.
They help in recruiting participants for studies and are involved in providing direct care for the participants.
Clinical research nurse salary can make use of their communication skills as well as their critical thinking skills gotten from their knowledge and experience in healthcare to further their career in this exciting way.
Know that future CRNs can speak to our 24/7 chat and phone advisors to request information on partial scholarships and payment plans for nurses.
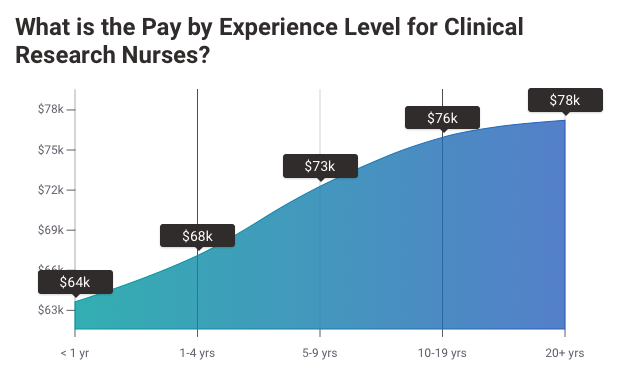
2. Clinical Research Nurse Salary
The average pay for a Clinical Research Nurse is $31.28 per hour.
MD Anderson Cancer Center Clinical Research Nurse salaries - $71,503/yr
Northwestern University Clinical Research Nurse salaries - $75,005/yr
NIH Clinical Research Nurse salaries - $77,331/yr
CLINICAL RESEARCH NURSE JOB Description
A clinical research nurse conducts scientific research on different aspects of human health like illnesses, pharmaceuticals, treatment plans and healthcare methods. Their major goal is to improve the quality of healthcare services that are administered to the patients.
3. How do I get Clinical Research Nurse Experience?
Experience don’t just jump on you, you have to get it by practice.CCRPS affords you an opportunity to acquire knowledge in clinical research, and not just knowledge but experience as well. Registering for the appropriate course will boost your knowledge base and as well you get experience of clinical research first hand.
As a clinical research nurse, you will be at the forefront of new medical discoveries, and help develop breakthrough cures and medical treatments. The work that you do during your career can help some patients live longer or better quality of life. You may be responsible for studying diseases and disorders, as well as developing new treatment plans. You will also help test new treatments and medications that could possibly change the way a disease or disorder is perceived.
The field of clinical research can be very rewarding and fulfilling. A good research nurse is dedicated to their work and ready to take on everything that the profession throws their way. If you’re looking to pursue a research nursing career, you should have an excellent understanding of the research process as well as the specialty area that you’re studying.
Excellent communication skills are also a must. You must be able to effectively communicate with scientists, physicians, researchers, patients, and corporate executives.
4. What Does a Clinical Research Nurse Do?
The duties of a research nurse will typically depend on their employer and role. Some research nurses may be responsible for studying diseases, while others may help create and improve new medications and other treatments.
clinical research nursing scope and Standards of Practice
Clinical research nurses can take up clinical research jobs in institutions like research organizations, pharmaceutical companies, universities, research laboratories, government agencies and teaching hospitals.
The work that a research nurse does is quite exhaustive and it includes;
They use their knowledge of the basics of clinical research in designing and implementation of research studies.
Observation of the procedures for patient treatment, collection and analyzing of data.
They report their research findings to the relevant authorities. They may also have to present their results at health conferences and publish them in journals.
They write grant applications in order to secure funds to carry out the research.
They render assistance in the process of recruiting study subjects.
They provide direct treatment for research participants.
Research nurses that study diseases and illnesses will often perform a great deal of research, both by studying previous findings and observing patients. They may be required to examine medical journals, for instance, as well as observe, study, and care for patients suffering from a particular disease.
They make decisions based on the observations made as to which patients are the best candidates for certain clinical trials. During clinical trials, the research nurse will administer medications or perform other treatment procedures, During this process, research nurses must closely monitor each patient’s progress. This includes documenting side effects, drug interactions, and the overall efficiency of the medication.
Aside from caring for patients, documenting and recording information during clinical trials are the most important responsibility that a research nurse has. The information and data gathered during the research must be compiled into reports and handed over to senior clinical researchers or specialists.
5. How Do I Become a Research Nurse?
Don’t expect to become a research nurse overnight. It's a lot of work and you are expected to undergo years of training and experience.
The clinical research nurse job is a competitive one and certificates are not just handed out to anybody. The conditions to be eligible to take the certificate exam is that you must be an experienced registered nurse and your experience must include having thousands of hours of experience in the area of clinical research.
How to Become a Registered Nurse (RN) in 2020 that contains everything a person pursuing a nursing job should know - responsibilities, education, salaries and more.
The first step toward becoming a research nurse is to obtain a proper education. You can start with a bachelor’s degree in nursing, although many employers prefer that their research nurses have master’s degrees or even doctoral degrees in their chosen specialty. During your schooling, classes in research and statistics are a must and are courses in your chosen area of expertise.
According to clinical research job websites, many research nurses have a MSN degree and some have a PhD in nursing. Many of them attain these degrees of education in order to give them an edge on getting clinical research positions. While studying, courses in statistics and research are mandatory.
There are two main certifications that clinical research nurses can get from the Association of Clinical Research Professionals (ACRP). You can get certification to become a certified clinical research associate or you can choose to become a certified clinical research coordinator.
Take courses from CCRPS and learn more on how to become a clinical research nurse.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
6. Clinical Research Nurse Requirements and Certifications & Nursing Cover Letter
A bachelor's degree in nursing does meet licensure requirements for graduates to become registered nurses (RNs), which qualifies individuals for the specialized certification. Bridge programs, such as an RN-to-Bachelor of Science in Nursing (BSN), require previous nursing education for admission. Nursing students complete traditional classroom courses, laboratory experiences, and a clinical practicum in a medical setting, which includes a hospital, assisted living facility, and long term care center.
For specific education in clinical research, trained RNs enroll in graduate certificate and degree programs. There students are introduced to case studies, ethical research practices, and financial matters affecting the design, implementation, and funding of clinical research trials. In a master's program, studies in research ethics point students towards ethical research practices, including a discussion on human rights, misconduct, and conflicts of interest. Graduate programs will also include quantitative research and a capstone project.
All RN-to-BSN programs will require an RN license to enroll. Master's and graduate certificates will need a bachelor's degree with sufficient prerequisite coursework in the field. In addition, they will need letters of recommendation or reference, a personal statement, and GRE scores.
Becoming a nurse researcher which is a highly specialized career requires an advanced degree and training in informatics and research methodology and tools. The initial step for these individuals, or for any aspiring advanced practice nurse, is to earn a Bachelor of Science in Nursing degree and pass the NCLEX-RN exam. Once a nurse has completed their degree and attained an RN license, the next step is to complete a Master's of Science (MSN) in Nursing program with a focus on research and writing. MSN courses prepare nurses for a career in research and usually include coursework in statistics, research for evidence-based practice, design and coordination of clinical trials, and advanced research methodology.
A TYPICAL JOB POSTING FOR A RESEARCH NURSE POSITION WOULD LIKELY INCLUDE THE FOLLOWING QUALIFICATIONS, AMONG OTHERS SPECIFIC TO THE TYPE OF EMPLOYER AND LOCATION:
MSN degree and valid RN license.
Experience conducting clinical research, including enrolling patients in research studies, Implementing research protocol and presenting findings.
Excellent attention to detail required in collecting and analyzing data.
Strong written and verbal communication skills for interacting with patients and reporting research findings.
For a person to practice nursing legally, acquiring of nursing credentials and certifications is very important. For instance, some nurses who achieve a master's degree (MSN) leave the patient care aspect of nursing, and practice in a more managerial role.
CRA JOB OPPORTUNITIES
If you choose to become a Clinical Research Associate (CRA), you will have a key role in the success of clinical trials. Most CRAs have a nursing background, like yours. You will be the primary contact and support for trial sites, ensuring that the study is conducted according to the protocol, ICH-GCP, regulatory requirements and standard operating procedures (SOPs).
The Clinical Research Associates also offers you the unique opportunity to have an exciting career in the research of drug and medical device development while making a difference in the lives of those around them.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Speak to our 24/7 chat and phone advisors to request information on partial scholarships and payment plans for nurses.