Top Medical Writing and Document Management Tools Directory and Reviews
Clinical research today runs on documentation — not just in volume, but in precision. Every clinical study report, protocol amendment, and investigator brochure must pass the litmus test of regulatory compliance. Inaccuracies, formatting errors, or version control gaps can halt submissions, trigger audit findings, and derail launch timelines. In this high-stakes environment, relying on general-purpose writing apps is not just inefficient — it’s risky.
That’s why specialized medical writing and document management tools have emerged as critical infrastructure across pharmaceutical, biotech, and CRO workflows. These tools go beyond word processors — offering automated formatting for ICH-compliant submissions, audit trails for inspection readiness, and real-time multi-author collaboration. This guide delivers a practical, in-depth look at the top clinical research documentation platforms — from Synchrogenix to Veeva — and shows how they help streamline submission readiness, boost productivity, and meet global regulatory demands. Whether you're a writer, data manager, or regulatory affairs professional, mastering these tools is no longer optional — it's a career imperative.
What Are Medical Writing and Document Management Tools?
Core Purpose and Capabilities
Medical writing tools and document management systems (DMS) play fundamentally different yet complementary roles in clinical research operations. Medical writing software focuses on regulatory document authoring—such as clinical study reports (CSRs), investigator brochures (IBs), and clinical trial protocols. These tools are optimized for accuracy, template consistency, ICH format adherence, and reducing formatting or structure-based rejections during submission.
For example, platforms like Synchrogenix support Clinical Document Architecture (CDA) and allow writers to auto-populate fields using clinical trial databases. This ensures faster document generation with minimal manual error. On the other hand, tools like PerfectIt act as intelligent grammar and compliance proofing engines—flagging deviations from EMA, FDA, or ICH stylistic requirements.
Meanwhile, document management tools are built for full lifecycle control. These DMS platforms enable version tracking, collaborative editing, automated approval flows, and secure cloud storage. Writers, reviewers, and regulatory teams can access audit logs, restore prior versions, and demonstrate document control during inspections. Features like real-time co-authoring, tracked changes, and e-signature compliance under 21 CFR Part 11 make DMS software indispensable across phases I–IV.
These tools are not optional extras—they are part of the compliance-critical infrastructure in regulated research environments.
Difference Between Writing Tools and DMS Platforms
While both writing and DMS tools support documentation, their technical objectives diverge sharply. Medical writing platforms are designed for drafting structured content that aligns with modules of the Common Technical Document (CTD). They prioritize formatting automation, built-in templates, medical terminology validation, and seamless alignment with regulatory expectations like ICH E3 and E6.
Document management systems, by contrast, focus on document lifecycle governance. Their value lies in audit readiness, controlled access rights, real-time versioning, and archiving. A well-integrated DMS reduces the burden on quality assurance teams, simplifies regulatory audits, and ensures a single source of truth for every submission document. The choice between them isn't either/or—clinical teams need both, integrated.
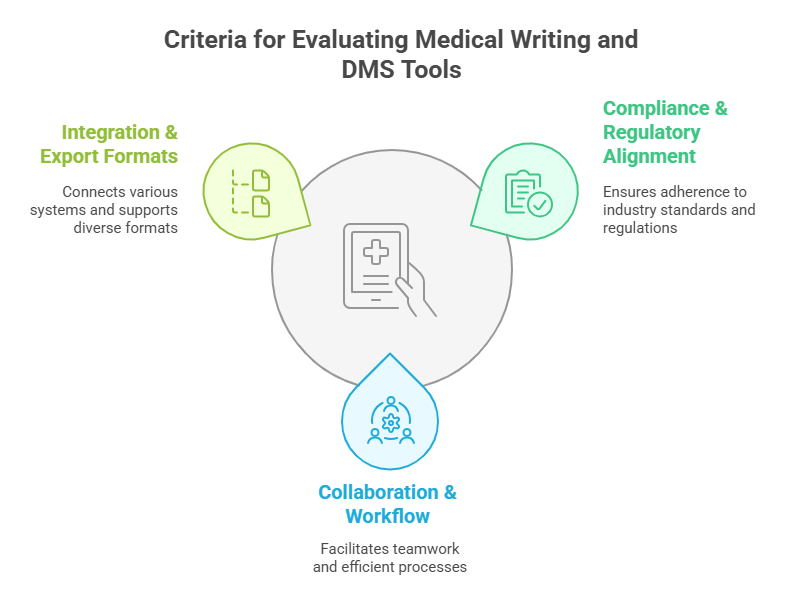
Criteria for Evaluating These Tools
Compliance and Regulatory Alignment
In clinical research, regulatory compliance is non-negotiable. The best tools are purpose-built to meet global health authority standards such as 21 CFR Part 11, ICH E3/E6, and the EMA IRIS submission gateway. These frameworks govern electronic signatures, audit trails, data integrity, and submission structure. Tools that lack these compliance layers risk rejection during submission, or worse, post-approval penalties for documentation discrepancies.
Leading platforms embed validation logic directly into the writing workflow. For instance, some flag non-compliant narrative phrasing or enforce document templates based on regulatory body requirements. DMS platforms ensure documents are timestamped, version-controlled, and locked during QA review—protecting teams during FDA inspections and sponsor audits.
What sets superior tools apart is pre-submission validation features. These simulate agency checks before actual filing, reducing chances of technical rejections through the EMA eCTD or US FDA’s ESG (Electronic Submissions Gateway). If a platform cannot demonstrate this level of compliance alignment, it’s not designed for real-world clinical operations.
Collaboration and Workflow Capabilities
Today’s multi-site global trials demand document tools that support parallel authoring, multi-role editing, and consolidated comment tracking. Clinical writers often work with statisticians, investigators, and regulatory leads—all of whom need synchronized access to working drafts and version history.
Top-tier tools allow real-time collaboration with granular access controls—so reviewers can annotate sections without overwriting critical content. Comment histories, tracked revisions, and automated email alerts are essential for both transparency and speed. They also simplify reconciliation workflows: when one author updates safety narratives, others get notified to check consistency across CSRs or DSURs.
Importantly, audit trails extend to comments and approvals. This level of traceability is often required during sponsor audits or GxP inspections to demonstrate who made changes, why, and when. If a tool can’t answer those questions, it’s not audit-ready.
Integrations and Export Formats
A clinical document tool is only as powerful as its interoperability. High-performing tools must integrate with key platforms such as clinical trial management systems (CTMS), electronic data capture (EDC), safety databases, and electronic Trial Master Files (eTMF).
For example, some medical writing software can ingest structured trial data from EDC systems to auto-generate safety narratives. Others support direct export in XML, DOCX, or eCTD-ready PDF formats—avoiding time-consuming reformatting before submission. These integrations reduce manual work and ensure documentation stays synchronized across trial systems.
Top Medical Writing Tools Reviewed
1. Synchrogenix (Certara)
Synchrogenix is a premier platform trusted by top 20 pharma companies for high-volume regulatory submissions. It provides AI-assisted authoring, automated CTD formatting, and ICH-guided templates. Built to handle large, multi-author writing projects like CSRs and INDs, it supports structured content reuse and offers pre-validation against FDA/EMA standards. Its integration with Certara’s regulatory strategy tools makes it a complete ecosystem for submission-ready document creation.
2. MedDRA Coding Tool (by MSSO)
This tool isn’t a general writer—it’s purpose-built for terminology accuracy. MedDRA (Medical Dictionary for Regulatory Activities) ensures all adverse events, indications, and medical terms are consistently coded across documents. Regulatory agencies expect standardized MedDRA use—and this tool automates mapping, flagging inconsistencies, and aligning with the latest MedDRA versions. Writers producing SAE narratives, DSURs, or IBs benefit from its deep terminology governance engine.
3. Trilogy Writing & Consulting
Unlike most platforms, Trilogy Writing blends tools with medical writing services. Their system uses internal quality checklists, modular templates, and real-time editing frameworks that support regulatory writers worldwide. While it's not a standalone SaaS platform, Trilogy’s interface is engineered to produce audit-proof documents in collaboration with their trained professionals. This hybrid model is ideal for sponsors needing expert co-authorship plus software stability.
4. PerfectIt + Microsoft Word Add-Ins
PerfectIt is a specialized plugin designed to enforce consistency and compliance in clinical trial documentation. It checks for abbreviation usage, capitalization, number consistency, and common ICH style requirements. While it doesn’t manage full workflows, it’s ideal for writers using Word to prepare CTD modules, narratives, or protocols. Combined with customizable style sheets, PerfectIt becomes a micro-QA engine for individual authors and lean medical writing teams.
5. WriteWise Medical Editor
WriteWise stands out for its AI-powered language refinement, helping non-native English speakers create documents that meet regulatory expectations. It checks for clarity, passive voice, jargon, and alignment with scientific tone standards. With an interface that mimics traditional word processors, WriteWise supports direct editing while suggesting compliance-focused improvements in real time. It’s especially useful for global teams contributing to global dossiers.
| Tool Name | Primary Purpose | Standout Features |
|---|---|---|
| Synchrogenix (Certara) | High-volume regulatory writing and submission prep | AI-assisted authoring, automated CTD formatting, FDA/EMA pre-validation, structured content reuse |
| MedDRA Coding Tool (MSSO) | Medical terminology standardization and coding | Automates AE coding, aligns with latest MedDRA versions, essential for DSURs and SAE narratives |
| Trilogy Writing & Consulting | Hybrid software + writing service for regulatory documents | Modular templates, expert co-authorship, real-time collaboration, audit-ready outputs |
| PerfectIt + Word Add-Ins | Consistency and compliance checks within Microsoft Word | Abbreviation rules, ICH formatting, style enforcement, micro-QA for lean writing teams |
| WriteWise Medical Editor | Language refinement for non-native English writers in clinical documentation | AI-powered clarity checks, scientific tone correction, live editing suggestions |
Top Document Management Systems for Medical Writers
1. MasterControl
MasterControl is a gold standard in regulated document management, widely used across pharma, biotech, and CROs. It offers complete document lifecycle automation—from draft creation and routing to review, approval, and archival. MasterControl is fully compliant with 21 CFR Part 11 and supports granular user permissions, automated workflows, and robust audit trails. It's particularly suited for clinical teams needing enterprise-level compliance and SOP alignment.
2. Veeva Vault RIM & QualityDocs
Veeva Vault combines regulatory information management (RIM) with high-performing document storage and review workflows. It enables submission-ready document preparation, collaborative authoring, and regulatory metadata tagging. Used by over 900 life sciences organizations, Vault ensures version traceability and submission package integrity. It also integrates with Veeva’s eTMF and CTMS tools, streamlining the end-to-end document ecosystem from protocol finalization to agency delivery.
3. Wingspan eTMF by IQVIA
Wingspan eTMF is a document repository designed specifically for clinical trial master files. It allows real-time tracking of regulatory documents, automates milestone-based filing, and ensures inspection readiness with prebuilt audit log features. Integrated with IQVIA’s broader clinical operations stack, Wingspan offers a seamless experience for sponsors managing multiple trials across regions. It’s particularly valuable for teams managing GCP compliance across global sites.
4. Montrium Connect
Montrium Connect is a cloud-based eTMF and document management platform tailored for small to mid-size biopharma companies. It offers configurable templates, automated alerts for upcoming deadlines, and GxP-compliant archiving. Montrium is praised for its ease of use, quick onboarding, and cost-effective compliance tools. Teams benefit from centralized dashboards showing document status, overdue items, and audit trails—all within a 21 CFR Part 11-validated environment.
5. ArisGlobal LifeSphere Docs
LifeSphere Docs by ArisGlobal supports full clinical and regulatory document management, with built-in compliance for global standards like EMA, FDA, and PMDA. It features role-based permissions, metadata tagging, and automated validation rules for structured documents. With AI-based suggestions for duplicate reduction and terminology alignment, LifeSphere Docs is a strong choice for mid-to-large companies needing cross-departmental coordination in document preparation and submission.
| Tool Name | Primary Purpose | Standout Features |
|---|---|---|
| MasterControl | End-to-end regulated document lifecycle management | 21 CFR Part 11 compliance, automated workflows, SOP enforcement, robust audit trails |
| Veeva Vault RIM & QualityDocs | Regulatory information management and collaborative authoring | Metadata tagging, submission integrity checks, integration with eTMF and CTMS |
| Wingspan eTMF by IQVIA | Trial master file automation and regulatory tracking | Milestone-based filing, audit logs, inspection readiness across multi-site trials |
| Montrium Connect | Cloud-based eTMF and DMS for small-to-mid biopharma | Configurable templates, automated alerts, GxP-compliant dashboards, quick onboarding |
| ArisGlobal LifeSphere Docs | Global clinical and regulatory document management | AI-driven document suggestions, metadata control, PMDA/EMA/FDA compliance features |
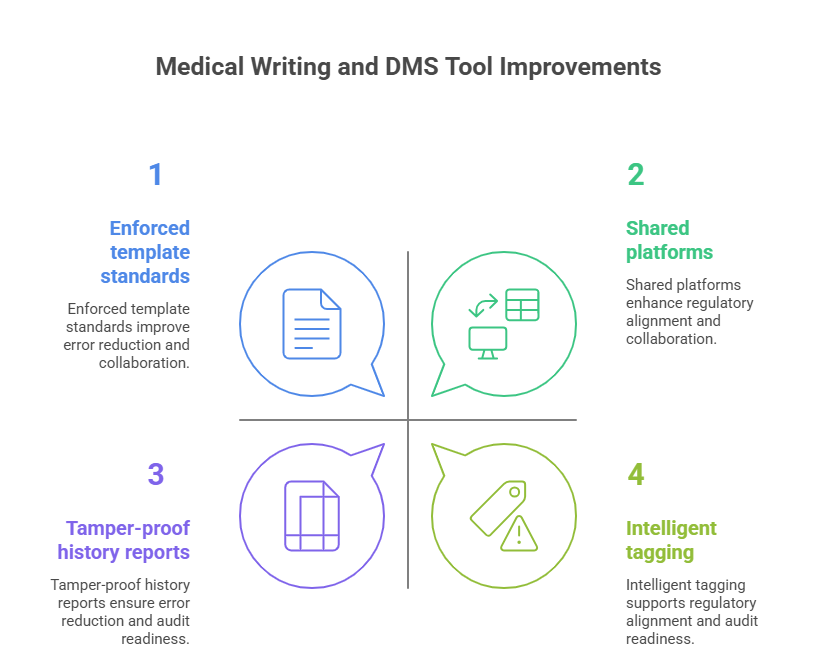
How These Tools Improve Trial Submission Accuracy
Reduce Manual Errors and Formatting Noncompliance
Submission failures often stem not from bad science, but from documentation defects. Minor inconsistencies in formatting, unapproved templates, or versioning mismatches can delay approvals or trigger rejection by regulatory bodies. That’s where medical writing and document tools deliver their first major impact: they eliminate manual rework loops and ensure adherence to submission-ready structures from the start.
Automated template enforcement, style checking, and glossary alignment help avoid formatting deviations. For example, platforms like Synchrogenix pre-validate section headers and data tables against ICH E3 and Module 2 specifications, reducing the chance of eCTD submission errors. PerfectIt add-ins can scan for abbreviations, font inconsistencies, or referencing errors—details that might slip through manual review.
Document management systems also reduce risks. Tools like Veeva Vault and MasterControl prevent outdated documents from entering the approval pipeline by locking prior versions, ensuring the team always works from a single, validated source. This alone eliminates a common root cause of regulatory audit findings.
Real-Time Regulatory Alignment and Traceability
The second core advantage lies in regulatory responsiveness. As global standards evolve—whether it’s a change in IRIS XML schema, FDA guidance, or EudraCT metadata rules—leading tools update workflows accordingly. Teams using legacy systems or manual tools often scramble to adapt, risking noncompliance.
By contrast, systems like LifeSphere Docs and Wingspan eTMF automatically align metadata, tag critical content fields, and flag nonconforming elements in real time. This level of intelligent validation enables teams to course-correct before submission—not after rejection.
Audit logs and document histories are another crucial benefit. During FDA or EMA inspections, auditors often demand a full trace of who edited what, when, and why. DMS platforms generate tamper-proof records showing document lineage—critical for demonstrating GCP compliance.
Finally, traceability also aids in cross-functional alignment. When medical writers, data managers, and regulatory leads collaborate using traceable systems, submission packages are more accurate, faster to compile, and less vulnerable to last-minute surprises.
How the Advanced Clinical Research Assistant Certification (CCRPS) Equips You for High-Stakes Medical Writing Roles
Why Certification Matters for Writers in Pharma/Biotech
Clinical research documentation isn’t just writing—it’s regulated evidence. Regulatory agencies like the FDA, EMA, and PMDA expect precise formatting, traceable metadata, and GCP-compliant authorship in every CSR, protocol, or IB. Writers who meet these standards are rare—and highly sought after. The Advanced Clinical Research Assistant Certification by CCRPS fills this skill gap with targeted, career-ready training.
Unlike general writing credentials, this program teaches professionals how to author submission-ready documents within the context of real clinical workflows. From document tracking and narrative consistency to ICH structure compliance, the certification prepares you for high-stakes deliverables that pass inspection the first time. It's designed for those aiming to specialize in roles like clinical writer, documentation coordinator, or regulatory assistant—not generic medical communicators.
This certification helps you move beyond grammar and style to regulatory submission fluency, which is exactly what CROs and sponsors need in today’s environment of accelerated trials and remote inspections.
Learn More About the Program
The Advanced Clinical Research Assistant Certification by CCRPS is a fully online, self-paced program that emphasizes clinical writing aligned with operational execution. Learners gain proficiency in:
Drafting CTD-ready Clinical Study Reports, protocols, and safety updates
Applying 21 CFR Part 11 and ICH E3/E6 compliance standards
Navigating documentation workflows in CTMS, EDC, and eTMF systems
Identifying and resolving issues that trigger submission rejections
Unlike theoretical programs, this certification is grounded in real-world document management. It walks learners through redline reconciliation, audit readiness documentation, and collaborative authoring frameworks used in sponsor-led trials. With CPD accreditation and global recognition, CCRPS certification carries weight in resumes, LinkedIn profiles, and vendor qualification assessments. Whether you're new to the field or formalizing years of experience, this program ensures you're trained not just to write—but to write what regulators approve.
Frequently Asked Questions
-
General writing focuses on clarity, grammar, and structure, but clinical research writing demands regulatory precision, scientific accuracy, and template compliance. Writers must adhere to ICH guidelines, CTD module structures, and ensure traceable data references across documents. For example, drafting a clinical study report (CSR) requires exact referencing of statistical outputs, proper integration of safety narratives, and formatting that aligns with FDA or EMA expectations. Unlike creative or journalistic writing, clinical writing is judged by regulatory bodies — not readers — which makes training in submission-driven documentation essential. Writers without this training often struggle with validation checks, leading to document rejections or costly revisions.
-
Tools like MasterControl or Veeva Vault create immutable audit trails, showing who edited each document, when, and why. This is a core requirement under 21 CFR Part 11, ensuring document traceability and accountability during FDA or EMA inspections. These platforms log every version, track electronic signatures, and provide metadata for each document state. During audits, sponsors often need to produce a full document history — these tools eliminate the guesswork. Instead of scrambling to locate versions or approvals, teams can instantly produce a timestamped trail, demonstrating compliance with GCP and regulatory expectations. This transparency significantly reduces audit risks.
-
Yes — especially when working in regulated environments. Document Management Systems (DMS) aren’t just about storage. They handle version control, track changes, manage approvals, and prevent unauthorized edits. In fast-paced trials, losing track of even a single protocol version can lead to submission errors or inspection findings. DMS platforms like Wingspan eTMF or LifeSphere Docs ensure that every stakeholder works off the correct, validated version — preventing conflicting data across CSRs, protocols, and appendices. For clinical writers, using DMS tools isn’t optional — it’s required for inspection readiness and internal consistency.
-
Yes. CCRPS is a globally recognized training provider, and its Advanced Clinical Research Assistant Certification is used by professionals in over 180 countries. The certification is CPD-accredited and built around real-world trial operations, making it highly relevant for global CROs and sponsors. Graduates work across roles like clinical documentation specialist, regulatory associate, and clinical trial coordinator. Because it emphasizes document quality, audit preparation, and ICH-compliant writing, it’s especially valuable for writers looking to specialize in submission-focused clinical documentation. It also adds credibility for those transitioning from general science writing into regulated environments.
-
Many of the top-tier tools are designed for cross-platform interoperability. For example, Synchrogenix and LifeSphere Docs integrate with systems like Medidata Rave (EDC), Oracle Siebel CTMS, or Veeva eTMF. These integrations allow data to flow automatically — such as patient counts, adverse event logs, or dosing schedules — into clinical documents. This minimizes transcription errors and reduces turnaround time. Document fields auto-populate with trial metadata, which supports real-time submission alignment. Integration isn’t just a convenience; it ensures that documentation and trial data stay synchronized for regulatory accuracy.
-
They support a wide range of submission-critical documents, including:
Clinical Study Reports (CSRs)
Investigator Brochures (IBs)
Protocols and amendments
Informed Consent Forms (ICFs)
Development Safety Update Reports (DSURs)
Common Technical Document (CTD) modules
Each document type has a distinct format and regulatory expectation, and these tools provide pre-built templates, validation logic, and tracking tools to meet those expectations. Without such systems, teams often rely on outdated formats or inconsistent narrative structures — both of which can delay approvals.
-
Absolutely — especially for teams submitting to global regulators. Tools like Montrium Connect or WriteWise offer cost-effective entry points with full compliance functionality. Even small errors in safety narratives, protocol headers, or submission metadata can delay approvals by weeks. For small teams without dedicated QA or regulatory staff, having a platform that enforces standardization and submission readiness is invaluable. These platforms don’t just save time; they reduce the risk of rework and failed audits — which can be far more expensive than the tool itself.
Final Thoughts
Clinical research success hinges on far more than study design—it depends on how well your trial is documented. In an environment where every protocol deviation, every adverse event, and every analysis set must be communicated with precision, medical writing and document management tools are no longer optional—they're mission-critical. Platforms like Synchrogenix, PerfectIt, Veeva Vault, and MasterControl empower teams to move faster while staying fully compliant with global regulations. These tools don’t just streamline workflows—they safeguard data integrity, reduce rework, and protect against costly submission delays.
And behind every tool is a person—trained, certified, and equipped to deliver. That’s where the Advanced Clinical Research Assistant Certification by CCRPS becomes indispensable. It closes the gap between software capability and human execution, giving professionals the training to not only use these systems—but to lead high-stakes clinical documentation with confidence.
Poll: Which Feature Matters Most in Medical Writing and DMS Tools?
| What’s the most valuable feature for improving trial submission accuracy? | |
| Compliance & Regulatory Alignment | |
| Collaboration & Workflow Automation | |
| Real-Time Traceability & Audit Readiness | |