What Does a Clinical Research Coordinator Do? Role & Duties
Ever wondered who ensures that groundbreaking medical treatments make it from the lab to your local hospital? Meet the Clinical Research Coordinator (CRC) – the unsung hero behind clinical trials. They juggle paperwork, manage patients, and make sure researchers don’t set things on fire (figuratively speaking). If you love science, organization, and a sprinkle of chaos, this role might just be for you!
What Is a Clinical Research Coordinator (CRC)?
A Clinical Research Coordinator (CRC) is the backbone of clinical trials. They work under the Principal Investigator (PI) to ensure studies are conducted ethically, efficiently, and according to regulations. Their responsibilities span from recruiting patients to maintaining compliance with regulatory agencies like the FDA. Without CRCs, clinical trials would be a logistical nightmare.
Key Responsibilities of a Clinical Research Coordinator
A CRC wears many hats, and their duties vary depending on the study and institution. However, their core responsibilities typically include:
1. Participant Recruitment & Screening
CRCs are responsible for identifying eligible candidates for a study based on predefined criteria. This involves:
Reviewing patient medical histories.
Conducting initial screenings.
Ensuring diversity and proper representation in clinical trials.
Addressing potential barriers to participation (e.g., travel, financial constraints).
2. Informed Consent Management
This is a critical ethical responsibility where CRCs ensure that participants:
Fully understand the nature, risks, and benefits of the study.
Have the opportunity to ask questions.
Provide voluntary, informed consent before participation.
3. Data Collection & Documentation
Accurate data collection is essential for the credibility of a trial. CRCs handle:
Recording patient responses, lab results, and any adverse effects.
Ensuring compliance with Good Clinical Practice (GCP) guidelines.
Using electronic data capture (EDC) systems to maintain organized records.
4. Regulatory Compliance
CRCs ensure all aspects of the clinical trial meet:
Institutional Review Board (IRB) approvals.
FDA and other regulatory guidelines.
Ethical standards for human research.
5. Patient Safety Monitoring
Patient well-being is a top priority, and CRCs:
Track and report any side effects or adverse events.
Provide support and guidance to participants.
Ensure that medical interventions comply with ethical standards.
6. Coordinating with Stakeholders
CRCs act as a bridge between different parties, including:
Sponsors who fund the trial.
Investigators and research teams.
Patients and regulatory bodies.
7. Handling Study Materials
CRCs manage essential clinical trial materials, such as:
Investigational drugs and placebos.
Laboratory samples.
Equipment and study documentation.
This role requires attention to detail, strong communication skills, and in-depth regulatory knowledge to ensure trials are conducted smoothly and ethically.
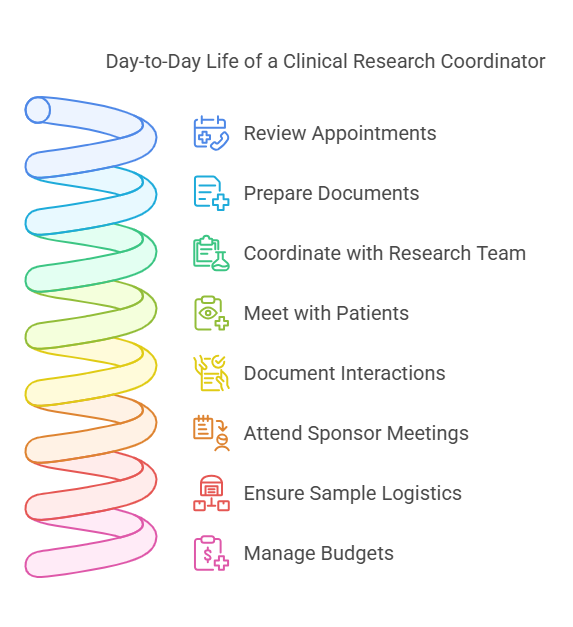
Day-to-Day Life of a CRC
A Clinical Research Coordinator’s workday is never dull. Their tasks range from administrative duties to patient interactions. Here’s a snapshot of what a typical day might look like:
Morning Routine
Reviewing upcoming appointments and preparing necessary documents:
CRCs start their day by checking the schedule to see which patients are coming in.
They prepare medical files, consent forms, and study-related materials.
Ensuring everything is in order prevents delays and ensures compliance.
Checking in with the Principal Investigator (PI) for study updates:
The PI is responsible for the clinical trial, and CRCs must align with their directives.
Any changes in protocols, patient statuses, or unexpected findings are discussed.
Coordinating with the research team to ensure all study materials are in place:
CRCs ensure that lab kits, investigational drugs, and necessary equipment are available.
They communicate with nurses, lab technicians, and regulatory personnel to confirm readiness.
Midday Tasks
Meeting with patients for screening, monitoring, or follow-ups:
CRCs interact with patients enrolled in clinical trials.
They conduct screenings to determine eligibility.
Monitoring includes checking vital signs, side effects, and adherence to study protocols.
Documenting all interactions in compliance with Good Clinical Practice (GCP):
Accurate documentation is crucial to ensure regulatory compliance.
CRCs enter patient responses, test results, and any reported symptoms into databases.
Attending sponsor meetings and regulatory briefings:
Sponsors fund the trials and require regular updates.
CRCs ensure the trial meets FDA and Institutional Review Board (IRB) standards.
Afternoon Responsibilities
Ensuring laboratory samples are sent to the correct testing facilities:
Blood, urine, or tissue samples need proper labeling and handling.
CRCs coordinate logistics with courier services and testing labs.
Managing budgets and grant documentation:
Clinical trials operate on strict budgets.
CRCs track expenses, submit invoices, and ensure financial compliance.
Submitting reports to ethics committees and regulatory bodies:
Ensuring the study aligns with ethical guidelines is crucial.
Reports on patient safety, adverse events, and protocol adherence are submitted.
Why Are Clinical Research Coordinators So Important?
A Clinical Research Coordinator (CRC) is a crucial figure in the execution of clinical trials. This section highlights the core reasons why CRCs are so important. Let’s break it down in depth:
1. Data Accuracy:
Clinical trials generate vast amounts of data, and any inaccuracies can compromise the validity of a study. CRCs ensure that data is:
Collected systematically.
Entered into databases correctly.
Verified against source documents (e.g., medical records).
They prevent issues such as missing data points, misinterpretations, or inconsistencies, all of which could lead to failed trials or regulatory disapproval.
2. Patient Safety:
One of the biggest ethical considerations in clinical research is patient safety. CRCs play a frontline role in monitoring:
Adverse events (AEs): Any unexpected symptoms or reactions.
Serious adverse events (SAEs): Severe medical occurrences that may require intervention.
Protocol adherence: Ensuring patients follow the trial’s guidelines to avoid risks.
CRCs act as patient advocates, ensuring they fully understand the study and receive proper medical care throughout.
3. Regulatory Compliance:
Clinical trials must adhere to strict regulations from organizations such as:
The FDA (Food and Drug Administration)
The EMA (European Medicines Agency)
The IRB (Institutional Review Board)
ICH-GCP (International Conference on Harmonisation - Good Clinical Practice)
CRCs ensure that:
All trial documentation is up to date.
Ethical guidelines are strictly followed.
Approvals and renewals are obtained as needed.
Trials remain compliant with evolving regulations.
4. Operational Efficiency:
CRCs coordinate multiple moving parts to keep clinical trials running smoothly. Responsibilities include:
Scheduling patient visits to fit within the required study timeline.
Ensuring investigational drugs are available and properly stored.
Coordinating with multiple stakeholders, including sponsors, doctors, nurses, and regulatory agencies.
Handling budget management for trial expenses like participant reimbursement and site resources.
Without CRCs, clinical trials would suffer from delays, inefficiencies, and increased costs.
The Consequences of Not Having CRCs
Without Clinical Research Coordinators, the clinical trial process would be chaotic and disorganized. Issues that might arise include:
✅ Delayed Drug Approvals – Trials would take longer, delaying life-saving treatments.
✅ Compromised Patient Safety – Without proper monitoring, trial participants could be at risk.
✅ Regulatory Non-Compliance – Leading to fines, shutdowns, or rejection of clinical studies.
Conclusion
The demand for skilled Clinical Research Coordinators is on the rise, especially with the expansion of clinical trials worldwide. Organizations like CCRPS offer specialized training and certification programs for aspiring CRCs. Whether you're starting or advancing in this field, becoming a CRC is a rewarding and impactful career choice!
Explore Courses for Clinical Research Career
Courses Available:
Frequently Asked Questions (FAQs)
-
Most CRCs have a background in life sciences, nursing, or healthcare. A Certified Clinical Research Coordinator (CCRC) certification is highly recommended.
-
Salaries vary by location and experience, but CRCs typically earn between $50,000 to $80,000 annually.
-
Attention to detail, organizational skills, regulatory knowledge, and strong communication are key.
-
Yes, CRCs frequently interact with patients, guiding them through trial procedures.
-
By adhering to GCP guidelines, securing informed consent, and working closely with ethics committees.