An Overview of Covance CRO Clinical Research I Covance Clinical Trials
The Funky Truth About Clinical Trials (And Why You Should Care)
Picture this: A group of scientists, lab coats fluttering dramatically, huddled over a microscope. Suddenly, one shouts, "Eureka! We’ve done it!" But before you celebrate their discovery, hold on—without clinical trials, that breakthrough is as useful as a chocolate teapot. Enter Covance Clinical Research—a powerhouse in the clinical research industry, ensuring that medical breakthroughs don’t just sit on a lab bench but reach the people who need them. Whether you're an aspiring clinical researcher, an industry veteran, or just someone who enjoys knowing how medicine gets from Petri dish to pharmacy shelf, this deep dive will give you everything you need to know about Covance clinical trials.
What is Covance Clinical Research?
Covance, now part of Labcorp Drug Development, is one of the world’s leading Clinical Research Organizations (CROs). They specialize in managing clinical trials, preclinical research, regulatory consulting, and laboratory testing—basically, everything needed to bring a drug from concept to consumer.
Covance plays a critical role in ensuring that new medical treatments are safe and effective. Their comprehensive clinical trial services span early-phase trials to post-marketing studies, working with pharmaceutical, biotech, and medical device companies worldwide.
To gain a deeper understanding of the scope of clinical research and its vast impact on modern medicine, explore this in-depth guide.
Why Covance Clinical Trials Matter
Ever taken an aspirin? Used allergy meds? Got a flu shot? If so, thank clinical trials. Covance is behind countless studies that make these everyday medicines safe and accessible. But beyond the basics, Covance trials push the boundaries of medicine, helping develop treatments for rare diseases, cancer, and even futuristic gene therapies.
The Clinical Trial Process at Covance
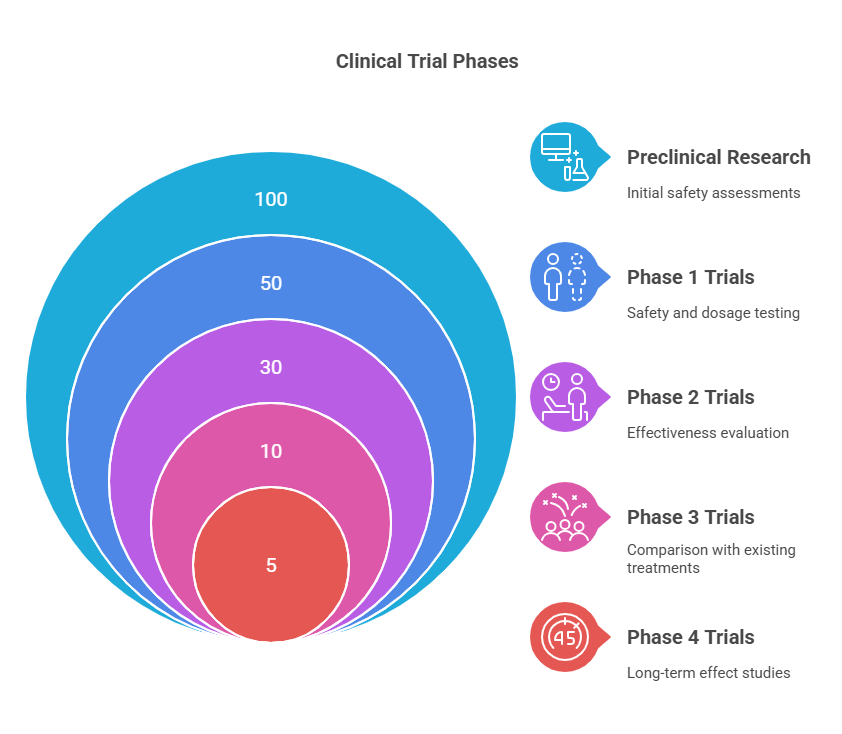
Clinical trials aren’t just random experiments—they follow a meticulously planned, step-by-step process to ensure safety and efficacy:
Preclinical Research: Before a drug reaches humans, it’s tested in labs (on cells and sometimes animals) to ensure it’s not dangerous.
Phase 1 Trials: Small groups of healthy volunteers test the drug for safety and dosage.
Phase 2 Trials: A larger group of patients with the targeted condition receives the drug to assess effectiveness.
Phase 3 Trials: Thousands of participants help determine how the drug compares to existing treatments.
Phase 4 Trials (Post-Marketing): Even after approval, the drug continues to be studied to monitor long-term effects.
Many people confuse the terms, but there are key differences between clinical research vs. clinical trials—understanding these distinctions is essential for anyone entering the field.
Covance’s Role in Cutting-Edge Research Areas
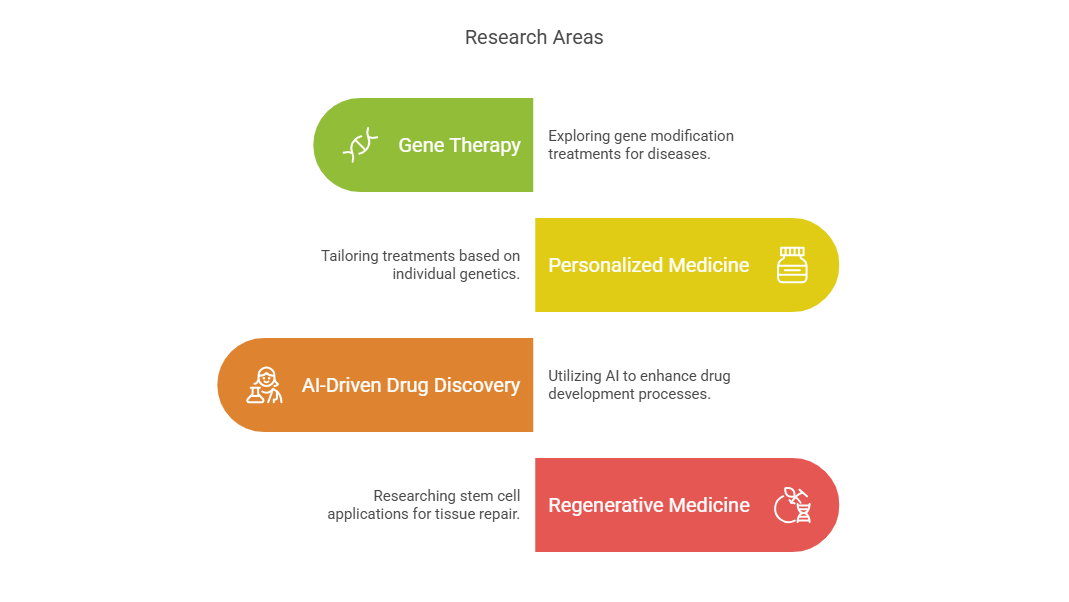
While Covance is known for conducting traditional clinical trials, it is also pioneering new research frontiers that could redefine modern medicine. Some of the groundbreaking areas Covance is actively involved in include:
Gene Therapy – Covance is exploring treatments that modify genes to cure hereditary diseases like cystic fibrosis and sickle cell anemia.
Personalized Medicine – Tailoring treatments to individual patients based on their genetic makeup and lifestyle.
AI-Driven Drug Discovery – Using artificial intelligence and machine learning to predict drug interactions and accelerate trials.
Regenerative Medicine – Covance is involved in studies using stem cells to repair damaged tissues and organs.
These cutting-edge fields not only make medicine more effective but also shorten the time it takes to bring treatments to market. If you're wondering about the best pathways to get into clinical research and build a successful career, here’s a comprehensive resource to help you start.
The Impact of Covance Clinical Research on Global Healthcare
Covance’s contributions to global healthcare extend beyond drug approvals. Their research has led to:
✅ New treatments for rare diseases that previously had no cure.
✅ Improved access to medications through collaborations with governments and non-profits.
✅ Faster pandemic response efforts, such as during COVID-19, where Covance played a role in vaccine and antiviral drug trials.
By running trials in over 100 countries, Covance ensures that life-saving treatments reach diverse populations worldwide.
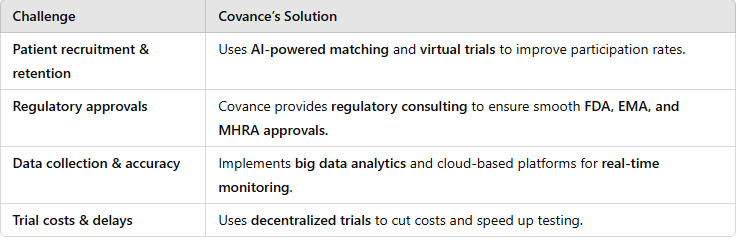
Challenges in Conducting Clinical Trials & How Covance Overcomes Them
Clinical trials are complex, with numerous hurdles that can delay or derail life-saving treatments. Some common challenges and Covance’s solutions include:
By staying ahead of these challenges, Covance keeps clinical trials efficient, ethical, and effective.
Covance’s Contribution to FDA Drug Approvals
Covance is a trusted partner in the drug development pipeline, helping secure regulatory approvals for some of the most widely used medicines today. Here’s what makes them stand out:
📌 Helped bring 90% of the top-selling drugs to market.
📌 Assisted in over 2,000 FDA approvals across various therapeutic areas.
📌 Provides regulatory strategy consulting to streamline approval processes.
Covance’s track record in FDA approvals speaks volumes about its reliability and expertise in clinical research.
10 Lesser-Known Facts About Covance Clinical Research
Covance was instrumental in developing the first-ever HIV treatment. Wikipedia
They conduct trials in over 100 countries worldwide. Wikipedia
Covance runs the largest Phase I trial unit in the U.S. Wikipedia
They provide regulatory consulting to navigate complex FDA requirements. Wikipedia
Covance uses AI and big data analytics to optimize trial designs. Wikipedia
They have a dedicated research division for rare diseases. Wikipedia
Covance helped bring over 90% of the top-selling drugs to market. Wikipedia
Their labs process over 500,000 samples daily. Wikipedia
Covance has been around since 1968! Wikipedia
They’re pioneers in decentralized and virtual clinical trials. Wikipedia
How to Get Involved in Clinical Research
If you're considering a career in clinical research or want to boost your expertise, certification programs can be a game-changer. Here are some of the best training options:
Good Clinical Practice (GCP) Certification – Master the ethical and regulatory standards for clinical trials.
Clinical Research Coordinator Certification – Learn the essentials of managing clinical trials.
Research Assistant Training Program – Get hands-on experience in trial management.
Clinical Research Management Training – Develop skills to oversee clinical trials and ensure regulatory compliance.
Clinical Research Associate (CRA) Certification – Train to monitor trials and ensure compliance with FDA regulations.
Pharmacovigilance Certification – Learn how to monitor drug safety and side effects.
Medical Monitor Certification – Specialize in the medical oversight of clinical trials.
Principal Investigator Certification – Lead clinical research projects with confidence.
Final Thoughts
Covance is a key player in the world of clinical trials, shaping the future of medicine one study at a time. Whether you're looking to participate in a trial, pursue a career in research, or simply understand how new treatments are developed, Covance’s work impacts every corner of modern healthcare.
For those eager to kickstart their journey in clinical research, check out the top-rated CCRPS courses and become a certified expert in this fast-growing industry. Join CCRPS today!
-
Covance manages clinical trials, preclinical research, regulatory affairs, and laboratory testing for new drugs and medical devices.
-
Visit the official Covance website or check ClinicalTrials.gov to find active studies.
-
Covance is known for its global reach, extensive laboratory services, and pioneering work in clinical research.
-
Yes, Covance was acquired by Labcorp and now operates as Labcorp Drug Development.
-
The duration varies by phase, but clinical trials can last from a few months to several years.
-
Yes, participants in Covance clinical trials are typically compensated for their time and travel expenses.
-
Covance conducts trials for a wide range of conditions, including oncology, cardiology, neurology, and rare diseases.
-
Earning a Clinical Research Coordinator Certification or Clinical Research Associate Certification from CCRPS is a great first step.