Essential GCP Exam Study Tips from Certified Experts
Achieving GCP certification is a significant milestone for professionals in clinical trials, but it requires focused preparation and strategic study techniques. Certified experts emphasize the importance of well-rounded study plans that combine theoretical knowledge with practical application. By following the right study tips and making the most of available resources, you can enhance your chances of passing the GCP exam on your first attempt.
This guide provides GCP exam study tips from certified professionals who understand the ins and outs of preparing for this essential certification. Whether you're new to clinical trials or looking to formalize your expertise, these tips will help you navigate the study process, balance practice with learning, and maximize your success. Let's dive into the strategies that will set you up for GCP exam success.
Why Study Tips Matter for GCP Certification
When preparing for the GCP certification exam, strategic study tips are crucial for maximizing your success. The exam evaluates your understanding of the Good Clinical Practice (GCP) guidelines, which are essential for ensuring ethical, safe, and scientifically sound clinical trials. Without a structured approach, it can be difficult to grasp all the concepts necessary for the exam, especially when there is so much material to cover.
Setting Realistic Study Goals
Setting realistic study goals is a cornerstone of effective exam preparation. Rather than attempting to master everything at once, break down your study material into manageable sections. Set clear, measurable objectives for each study session, such as mastering a specific GCP topic or completing a set number of practice questions. This approach not only keeps you on track but also ensures that you understand each concept before moving forward.
Balancing Study with Practice
Studying for the GCP certification exam requires more than just reading textbooks. It's equally important to practice applying your knowledge. Active recall, through practice tests and case studies, helps reinforce key concepts. As you study, set aside time to test yourself on the material, focusing on the areas where you’re weakest. This balance between study and practice builds both your knowledge and your confidence, giving you the best chance of passing the exam on your first attempt.
Top Study Tips for GCP Certification Exam
Mastering the GCP certification exam requires a smart approach to studying. The following tips, based on expert advice, will help you prioritize your time and effort, ensuring you are well-prepared for the test. By focusing on the key areas of the exam and adopting effective study techniques, you can confidently approach the certification process.
Focus on Key GCP Areas
Not all topics in the GCP exam are weighted equally. Focus on high-priority areas like informed consent, trial monitoring, and data integrity. These sections are frequently covered in the exam and are critical for ensuring that clinical trials adhere to ethical and regulatory standards. Make sure to understand these topics deeply, as they are foundational to the Good Clinical Practice guidelines.
Active Learning Techniques
Active learning is one of the most effective study methods for preparing for the GCP exam. Instead of passively reading materials, engage with the content through techniques like mind mapping, flashcards, and group discussions. These methods help reinforce your understanding and retain information longer. Additionally, teaching someone else what you've learned can solidify your knowledge and identify gaps in your understanding.
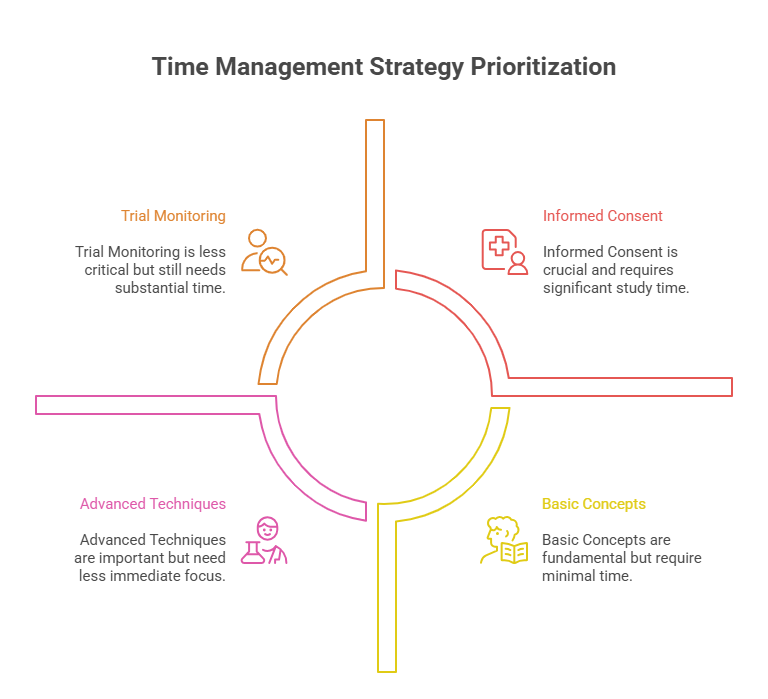
How to Manage Your Time During GCP Exam Preparation
Time management is one of the most important aspects of preparing for the GCP certification exam. With a large amount of material to cover, organizing your study schedule efficiently can make all the difference. The key to success is balancing focused study sessions with ample review and practice, ensuring that you cover all the necessary topics without feeling overwhelmed.
Creating a Timely Study Schedule
Start by mapping out a study schedule that works for your individual pace. Allocate specific blocks of time each day to study, making sure to include regular breaks to keep your mind fresh. Consistency is key, so stick to your schedule and gradually build up your knowledge. Use tools like calendars or productivity apps to track your progress and adjust the plan as needed. Prioritize topics based on your current understanding and the GCP exam syllabus.
Prioritizing Critical Exam Topics
When you’re pressed for time, focus on the critical exam topics that carry the most weight, such as informed consent procedures, trial oversight, and regulatory compliance. Allocate extra study time to these areas while ensuring that you also cover other topics to avoid neglecting any section. Understanding the most frequently tested areas will help you maximize your study time and boost your chances of success.
Essential Study Resources for GCP Exam
Using the right study resources is crucial for ensuring that you’re fully prepared for the GCP certification exam. With so many materials available, choosing high-quality, comprehensive resources will streamline your study process and help you focus on the most relevant content. Here are the best study resources to guide you through your preparation.
Recommended Books and Online Courses
Books and online courses are excellent resources for structured learning. The ICH-GCP guidelines should be your primary reference, but there are also various textbooks and online courses tailored to the GCP certification. Look for GCP study guides and certification prep books that offer summaries, detailed explanations, and practice questions. Online courses often include video lectures, quizzes, and discussion forums, making them an interactive and convenient way to reinforce your understanding.
Using Practice Tests Effectively
Practice tests are one of the most valuable tools for GCP exam preparation. They help you familiarize yourself with the exam format and question types, as well as identify areas where you need improvement. Set aside time for regular mock exams to test your knowledge and refine your exam-taking strategies. Reviewing your results will help pinpoint weak spots, allowing you to focus your study efforts on those areas before the real exam.
| Resource | Description |
|---|---|
| Official GCP Guidelines | The ICH-GCP guidelines are the primary source of information for clinical trials, outlining the essential principles and practices of Good Clinical Practice. These guidelines are a must-read for understanding the regulatory requirements and ethical considerations in clinical research. |
| Books on GCP Certification | Books specifically tailored to GCP certification provide a structured learning experience, covering topics from trial monitoring to data integrity. Look for updated editions that include recent changes in regulatory frameworks and clinical trial best practices. |
| Online Courses | Online GCP certification courses are convenient, interactive, and often include video lectures, quizzes, and practice exams. These courses are designed to provide an in-depth understanding of GCP concepts and allow for flexible learning at your own pace. |
| Practice Tests | Practice tests simulate the GCP exam environment, providing valuable feedback on your knowledge and exam-taking strategy. Regular practice will help you identify weak areas, manage time effectively, and familiarize yourself with question formats. |
| Industry Journals and Articles | Stay updated with the latest GCP regulations and trends in clinical research by reading journals like the Journal of Clinical Research and articles published by regulatory bodies. These resources provide real-world case studies and insights into current practices and innovations in the industry. |
Study Groups and Community Support for GCP Exam Success
Connecting with peers and experts can significantly boost your preparation for the GCP certification exam. Study groups and online communities offer opportunities to share resources, discuss difficult topics, and gain insights from others who are also working toward certification. Learning from others can enhance your understanding and keep you motivated throughout the study process.
Joining a study group allows you to engage in discussions about key concepts, ask questions, and clarify any doubts you may have. It’s an excellent way to deepen your knowledge of complex topics like data integrity and trial monitoring. Additionally, having a community to support you keeps you accountable, helping you stay on track and focused during your preparation.
Whether online or in-person, study groups can provide a sense of camaraderie and reassurance, helping to reduce the stress of preparing for the exam. Engaging with GCP certification forums and communities can also offer access to exclusive study materials, real-world scenarios, and expert tips that will aid you in mastering the GCP exam content.
| Benefit | Description |
|---|---|
| Peer Interaction | Participating in study groups allows you to exchange knowledge, ask questions, and discuss complex GCP topics. Engaging with peers provides fresh perspectives and can help clarify difficult concepts, making the study process more effective and enjoyable. |
| Accountability | Being part of a study group helps you stay motivated and accountable. Group members can encourage you to stick to your study schedule, discuss progress, and provide support when facing difficult topics. This accountability increases the likelihood of consistent study habits. |
| Expert Insights | Online communities and forums, such as GCP-focused LinkedIn groups or clinical trial forums, allow you to interact with industry experts. These professionals can share their real-world experiences and offer valuable tips on preparing for the GCP exam based on their own success stories. |
| Access to Additional Resources | Many study groups and forums provide members with access to exclusive study materials, practice questions, and industry reports. This allows you to supplement your learning with additional resources that might not be readily available through standard textbooks or online courses. |
| Increased Confidence | Engaging with a supportive group of peers and experts helps reduce exam anxiety and boosts confidence. With continuous interaction, you will feel more prepared and less stressed about the challenges of the GCP exam, improving your overall chances of success. |
How the CCRPS GCP Certification Can Help You Pass the GCP Exam
Enrolling in the CCRPS GCP certification program is one of the most effective ways to ensure success in the GCP exam. The CCRPS GCP certification provides a comprehensive, structured curriculum that aligns directly with the ICH-GCP exam content. By completing this certification, you will gain a deep understanding of Good Clinical Practice principles and learn how to apply them in real-world clinical trial settings.
The CCRPS GCP certification includes study materials, practice exams, and expert guidance that help reinforce key concepts such as informed consent, trial monitoring, and data integrity—all of which are heavily tested in the GCP exam. This certification not only prepares you for the exam but also builds your confidence and ensures you are familiar with the most up-to-date regulatory standards and industry practices.
Additionally, CCRPS offers ongoing support throughout your preparation, providing you with a clear path to success. The combination of structured learning, real-world applications, and access to expert resources makes the CCRPS GCP certification an invaluable tool in your exam preparation journey.
Frequently Asked Questions (FAQs)
-
GCP certification ensures professionals understand and can apply Good Clinical Practice (GCP) standards in clinical trials. It’s essential for maintaining ethics, data integrity, and regulatory compliance in clinical research. This certification is globally recognized and often required for clinical research positions, offering a competitive edge in the job market.
-
Preparation time for the GCP exam varies depending on prior knowledge. On average, candidates need 2-4 weeks of focused study time, with 1-2 hours per day. A structured study plan and practice tests can help expedite learning and improve retention, allowing you to be ready for the exam within a month.
-
The GCP exam covers key topics like informed consent, trial monitoring, data management, and ethical considerations. Additionally, you’ll need to understand regulatory requirements, participant safety, and study protocols. A thorough grasp of these areas is essential to passing the exam and applying GCP guidelines effectively.
-
Start by focusing on core topics such as trial monitoring and data integrity. Use active learning methods, including practice exams and case studies, to reinforce your knowledge. Additionally, enrolling in a GCP certification course will provide structured learning and expert insights, ensuring comprehensive preparation.
-
Practice exams simulate the GCP exam environment, helping you familiarize yourself with the types of questions and time constraints. They highlight weak areas and allow you to refine your knowledge before the actual exam. Consistent practice improves recall and helps manage exam anxiety by boosting confidence.
-
Recommended resources include the ICH-GCP guidelines, specialized textbooks, online courses, and practice tests. These materials provide in-depth coverage of exam topics, including trial protocols and regulatory compliance. Ensure that your resources are up-to-date to align with the latest standards and regulations.
-
While GCP certification isn’t always mandatory, it is highly recommended and often required for clinical research professionals, particularly those involved in data collection, monitoring, or regulatory oversight. GCP certification is a recognized qualification that demonstrates your commitment to ethical and regulatory standards in clinical trials.
Conclusion: Setting Yourself Up for GCP Exam Success
Preparing for the GCP certification exam requires a strategic approach, dedication, and the right resources. By following expert study tips, focusing on key areas like informed consent, trial monitoring, and data integrity, and utilizing top study materials like the CCRPS GCP certification course, you can confidently tackle the exam.
Remember, time management is crucial, so create a study schedule and prioritize critical exam topics. Actively engaging with practice tests and learning from study groups will also enhance your preparation. With the right mindset, tools, and expert support, you’re well on your way to achieving success on the GCP exam.