How Clinical Trials and Data Management Impact Research Outcomes?
Clinical trials are the backbone of medical advancements, providing the necessary data to develop new treatments, medications, and healthcare strategies. However, the success of these trials does not only depend on the execution of the experiments but also on how data is managed throughout the process. Data management plays a pivotal role in ensuring accurate, reliable, and usable information, which is critical to making informed decisions that ultimately shape research outcomes.
This blog will delve into the intricate relationship between clinical trials and data management, highlighting why data management is crucial to research outcomes. Additionally, we'll explore how data accuracy directly influences clinical trial results and subsequent decisions.
The Relationship Between Clinical Trials and Data Management
At the heart of every clinical trial is data. Data management in clinical trials involves the collection, cleaning, processing, and analysis of data from various sources such as patient reports, medical devices, and lab results. Efficient data management ensures that the information collected is accurate, consistent, and accessible for further analysis. Poor data management can result in errors, inconsistencies, and delays in clinical trials, which can compromise the integrity of the research.
1. Data Collection in Clinical Trials
Data collection in clinical trials includes a wide range of variables such as patient demographics, medical histories, drug dosages, side effects, and outcome measures. The accuracy and completeness of this data are crucial because clinical trials are designed to evaluate the safety and efficacy of treatments. Any missing or erroneous data could skew the results and lead to misleading conclusions. Data management systems help standardize the collection process, reducing human error and ensuring consistency across multiple trial sites.
2. Data Cleaning and Validation
Once the data is collected, it undergoes a process known as data cleaning, where errors or inconsistencies are identified and corrected. This process is essential because clinical trials often generate large volumes of data from diverse sources. Without proper cleaning and validation, the data could contain inaccuracies, such as duplicate entries or incorrect values, that can affect the outcomes of the trial. Data management tools and software are designed to streamline this process, making it more efficient and accurate.
3. Data Integration and Accessibility
In large-scale clinical trials, data is often collected from multiple sites and sources. Integrating this data into a central database is crucial for analysis. An effective data management system allows seamless integration of data, ensuring that it is stored in a structured format that can be easily accessed and analyzed. This accessibility is important for researchers who need real-time insights into the trial’s progress and outcomes.
Why Managing Data Effectively Is Critical to Research Outcomes?
Data is the foundation upon which decisions are made in clinical trials. Proper data management not only ensures the accuracy and reliability of the information but also significantly impacts the success of the trial and, ultimately, patient safety and treatment efficacy.
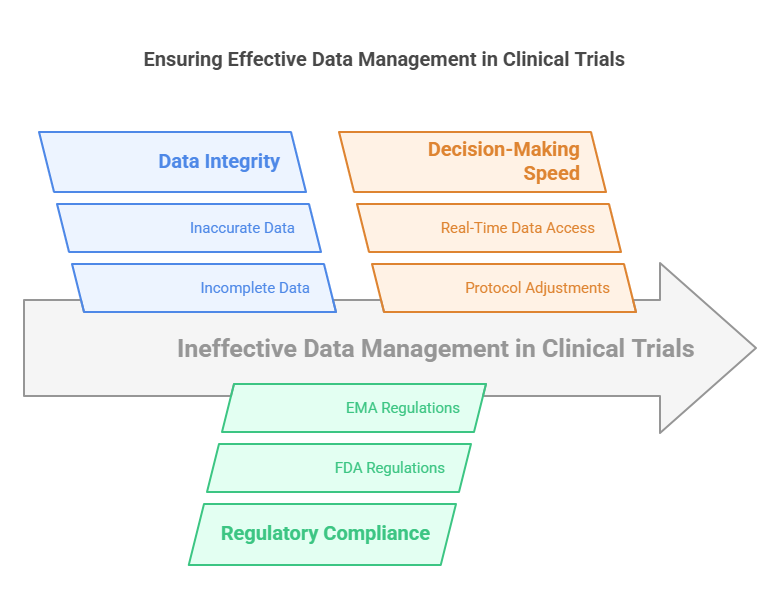
1. Data Integrity and Trial Validity
The integrity of data in clinical trials is paramount. Clinical trials often involve testing life-saving medications or treatments, and any compromise in the data could lead to catastrophic results. If data is incomplete or inaccurate, it can lead to incorrect conclusions about the efficacy or safety of a treatment. By managing data effectively, researchers can ensure that the trial’s findings are valid, making the trial’s results more trustworthy and credible.
2. Regulatory Compliance
Clinical trials are subject to stringent regulations imposed by regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). These agencies require that data collected during clinical trials is managed according to strict guidelines to ensure its reliability and safety. Effective data management helps clinical trial sponsors comply with these regulations, avoiding potential legal issues or delays in drug approval processes.
3. Faster Decision-Making
The ability to make informed decisions quickly is critical in clinical trials. Researchers need to analyze data as it is collected and make adjustments to the trial protocol if necessary. Effective data management tools provide real-time access to data, enabling researchers to make decisions faster and more accurately. This leads to improved trial outcomes and quicker responses to unexpected issues or trends.
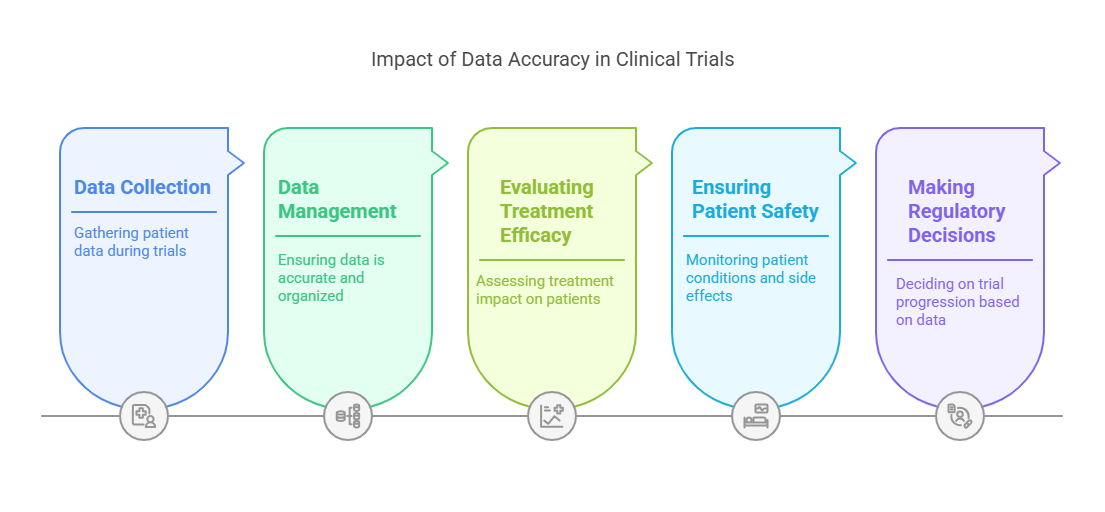
How Data Accuracy Influences Clinical Trial Results and Subsequent Decisions?
Accurate data is the cornerstone of effective decision-making in clinical trials. Whether it’s adjusting dosages, identifying side effects, or determining the efficacy of a treatment, data accuracy directly impacts every aspect of the trial. Poor data management can lead to incorrect conclusions and, in some cases, may even result in halting the trial prematurely.
1. Evaluating Treatment Efficacy
One of the primary goals of clinical trials is to evaluate the efficacy of a treatment. This requires precise measurement of the treatment’s impact on patients across various stages of the trial. Without accurate data, researchers may fail to identify subtle yet critical effects of the treatment. Effective data management allows researchers to track patient progress and identify patterns that may not be immediately obvious, ensuring that the trial results reflect the true efficacy of the treatment.
2. Patient Safety
Patient safety is always the top priority in clinical trials. Accurate data on patient conditions, side effects, and treatment responses is crucial for ensuring that treatments are safe. Any discrepancies or errors in data could result in incorrect assessments of a treatment’s safety profile. Data management systems that ensure data accuracy help protect patients by flagging potential safety concerns early in the trial process.
3. Regulatory and Ethical Decisions
Data also plays a key role in making regulatory and ethical decisions throughout the trial. For example, if the data shows that a treatment is causing severe side effects, the trial may need to be paused or terminated. Conversely, if the data shows that the treatment is effective, the trial may move forward more quickly. Accurate data ensures that these decisions are based on factual information rather than assumptions, leading to better outcomes for patients and a more streamlined regulatory process.
10 Lesser-Known Facts About Data Management in Clinical Trials
Real-Time Data Collection is Now Possible: With advancements in technology, clinical trials now allow for real-time data collection, leading to faster decision-making.
Data Monitoring Can Predict Outcomes: Advanced data analytics can predict clinical trial outcomes before they are officially concluded, allowing for quicker interventions.
Artificial Intelligence (AI) is Revolutionizing Data Management: AI and machine learning are now being used to analyze clinical trial data faster and more accurately than ever before.
Blockchain is Enhancing Data Security: Blockchain technology is being integrated into clinical trials to ensure the security and integrity of the data collected.
Wearable Devices Are Shaping Data Collection: Wearables are increasingly being used in clinical trials to collect real-time data on patient health metrics, reducing reliance on self-reporting.
Patient-Reported Data is Becoming More Prominent: More trials are incorporating data directly from patients, providing a more holistic view of treatment effects.
Cloud-Based Systems Are Reducing Costs: Cloud-based data management systems are making it cheaper and easier to store and access clinical trial data.
Data Analytics Are Improving Trial Designs: Advanced analytics are helping design better clinical trials, reducing waste and improving the accuracy of results.
Mobile Apps Are Making Participation Easier: Mobile apps are being used to track patient adherence to trial protocols, improving data quality and engagement.
Data Management Systems Are Becoming More User-Friendly: Modern data management systems are increasingly user-friendly, allowing non-experts to easily navigate complex data.
Related Blogs
What is Clinical Trial Management?
The Basics of Clinical Trial Data Management
Explore Courses for Clinical Research Career
Courses Available:
Conclusion
In conclusion, effective data management is crucial to the success of clinical trials, directly impacting the accuracy of results and decision-making. By ensuring data integrity, improving decision-making speed, and supporting regulatory compliance, strong data management practices help researchers achieve reliable outcomes. As technology continues to evolve, the role of data management in clinical trials will only grow more significant, paving the way for safer, more efficient healthcare advancements. At CCRPS, we are dedicated to providing innovative data management solutions that enhance the quality and success of clinical trials.
Frequently Asked Questions (FAQs)
-
Data management ensures the accuracy, consistency, and reliability of the data collected, which is crucial for making informed decisions and maintaining the integrity of the trial.
-
Poor data management can lead to inaccurate results, affecting the trial's conclusions and potentially compromising patient safety and treatment efficacy.
-
AI, machine learning, blockchain, and cloud-based systems are revolutionizing how data is managed, analyzed, and stored in clinical trials.
-
Real-time data collection systems allow researchers to monitor patient data as it’s being generated, enabling faster decision-making and more accurate assessments of treatment efficacy.
-
Patient-reported data provides a more comprehensive view of a treatment’s effects, contributing valuable insights into patient well-being and treatment outcomes.