Importance of a Drug Safety Pharmacovigilance Certificate Program
Ensuring patient safety through the detection, assessment and management of the adverse effects of drugs is only possible through drug safety and pharmacovigilance. This has become more important given the current focus on drug safety in the healthcare sector, and therefore obtaining a Pharmacovigilance Certification is important for anyone wishing to contribute to the safety of pharmaceutical products. Adverse drug reactions (ADRs) are frequent, prevenable and contribute significantly to morbidity and mortality. It is crucial to provide essential education and training for healthcare professionals who are involved in drug prescription and administration to avoid these risks.
Drug Safety Pharmacovigilance Certificate Program
Fig1.1 Importance of drug safety and pharmacovigilance
Adverse drug events are frequent, many are preventable, and are a leading cause of mortality and morbidity. Pharmacovigilance is the science of monitoring, reporting and preventing the adverse effects of drugs. It is important to include education of health care professionals in the use of drugs as part of the measures to avoid and control these adverse effects. To do this, we must focus on three pivotal aspects:
•Awareness
•Knowledge
•Reporting
As from 2025, with increasing complexity of regulatory requirements and data management techniques, drug safety certification programs are becoming more important to ensure that professionals are ready to meet the increasing challenges of this field. For those who wish to enter the field of pharmacovigilance, proper training and certification is a way to move up the career ladder in this industry.
Global drug safety and pharmacovigilance
With the increasing awareness of the patient safety movement across the world, the role of pharmacovigilance has become more significant. The burden of drug related diseases is still a major problem in the healthcare systems of many countries of the world. A meta-analysis conducted in the USA which is quite widespread, reveals that drug related disease is one of the major causes of death in many people. This makes pharmacovigilance an important area of focus.
Pharmacovigilance divides into three key parts:
Identify drug-related problems
Contribute to reducing these problems
Increase knowledge about these issues
This is an effective way of preventing drug related adverse effects through Pharmacovigilance training. Pharmacovigilance is a new and a small field and it is not a very well established academic program. The current training programs of pharmacy, clinical medicine and clinical pharmacology do not provide adequate training needed for pharmacy vigilance. Few universities offer special training in pharmacovigilance, the reason being that it covers a wide range of topics, and the International Society of Pharmacovigilance (ISP) offers training programs for special pharmacovigilance.
For more details about how to advance your career in pharmacovigilance or drug safety, check out our Guide on Becoming A CRA in 2025.
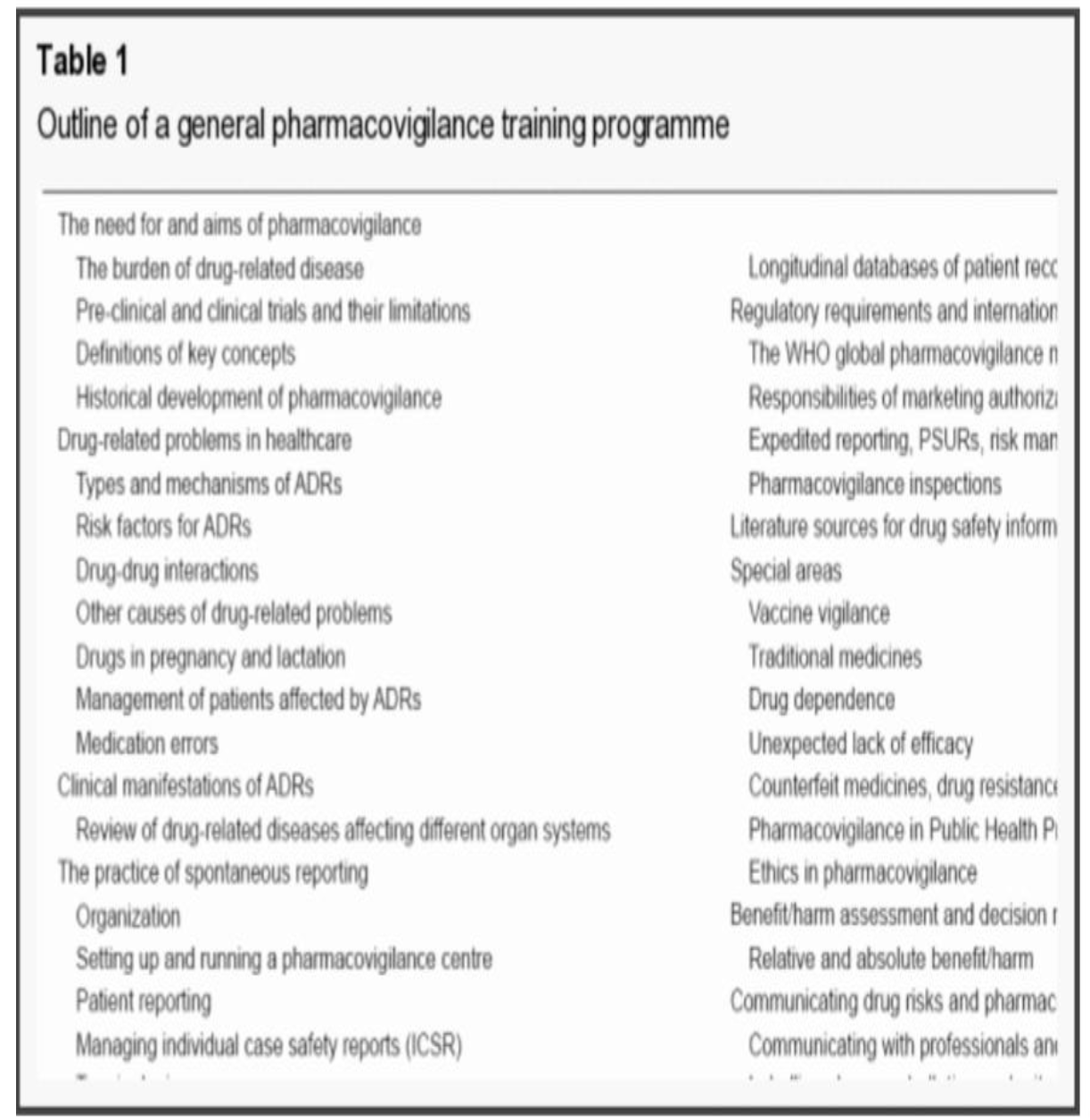
Table 1.2 General topic for training of all pharmacovigilance employee
Two types of training occur in pharmacovigilance
There are two main types of training in pharmacovigilance:
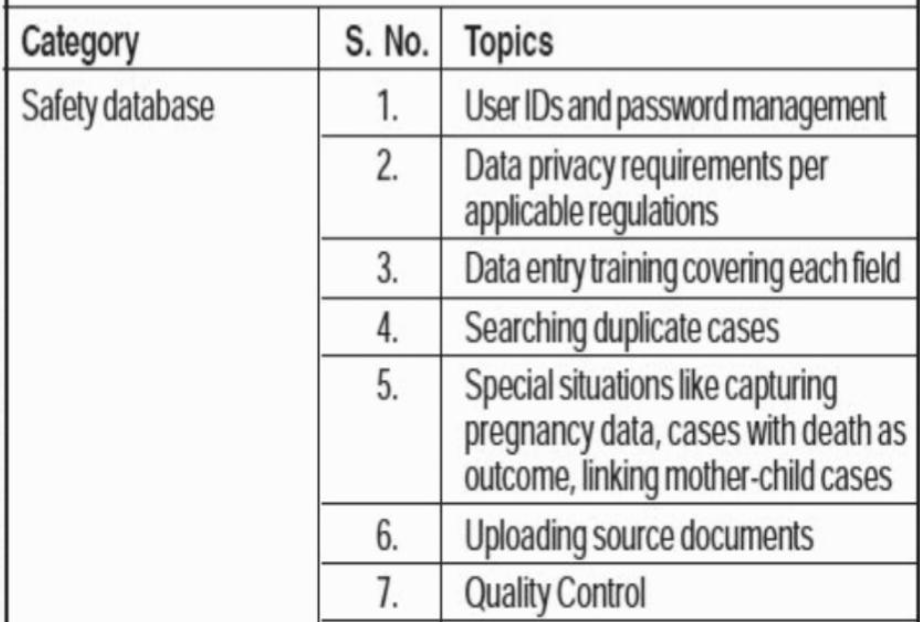
Training for pharmacovigilance staff who will work on computer systems.
Training for other company employees who may not directly work with pharmacovigilance systems but need awareness of the processes involved.
Training programs should be tailored to the particular job role for which they are intended. For instance, those who work in data management and computer systems need very specific training in the technologies and regulatory requirements of drug safety
New employees should receive appropriate training as soon as they join the company. While face-to-face training is ideal, geographical differences often make this difficult. To address this, various training modalities such as self-training, video conferencing, phone calls, and web-based classrooms are utilized.
With the increasing importance of pharmacovigilance in 2025, advanced pharmacovigilance training programs are now more accessible than ever. These programs allow for flexible learning while providing the expertise necessary to manage drug safety challenges effectively.
Effective training can be achieved through careful planning and identifying the specific needs of employees, matching those needs with the objectives of the company. Many pharmacological institutes organize pharmacovigilance seminars to emphasize the importance of drug safety and pharmacovigilance training.
For more information on clinical research careers and to explore opportunities in clinical research professionals worldwide, visit CCRPS.
Explore These Courses for Enhanced Learning:
Training of pharmacovigilance staff who will work on computer systems. To deepen your knowledge, check out the Pharmacovigilance Certification.
The topic of the training should cater to job requirements, providing detailed education for pharmacovigilance employees. Consider the comprehensive Pharmacovigilance Certification.
For new employees, overcoming geographical differences in training is crucial. Online courses like the Advanced Clinical Research Project Manager Certification and the Medical Monitor Certification are excellent resources.
Interested in coordinating clinical research? The Clinical Research Coordinator course provides essential training.
Explore the role of a Clinical Research Associate with the CRA course.
For those involved in international health care compliance, the ICH-GCP course offers critical guidelines and practices.
Assist in clinical trials more effectively by undertaking the Clinical Trials Assistant Training.
Physicians aiming to become principal investigators can benefit from the Advanced Principal Investigator Physician Certification.