Pharmacovigilance Certification Exam Comprehensive Prep Guide
Pharmacovigilance is more than monitoring drug safety—it’s a mission-critical discipline that protects lives across global healthcare systems. Earning a pharmacovigilance certification proves you're not only fluent in risk management plans, MedDRA, and post-marketing surveillance—but also ready to lead in regulatory-compliant safety practices. The exam isn’t just technical; it tests real-world decision-making in high-stakes clinical scenarios.
This guide is built to give you pure prep value—no filler, no surface-level tips. Whether you’re navigating ADR reporting systems, signal detection protocols, or region-specific regulations like FDA and EMA, your focus needs to be tight and tactical. We'll break down what to expect on the exam, how to structure your study plan, and which resources actually yield results. If you're serious about passing, this guide is your blueprint.
What to Expect on the Pharmacovigilance Exam
Structure, Duration, and Domain Breakdown
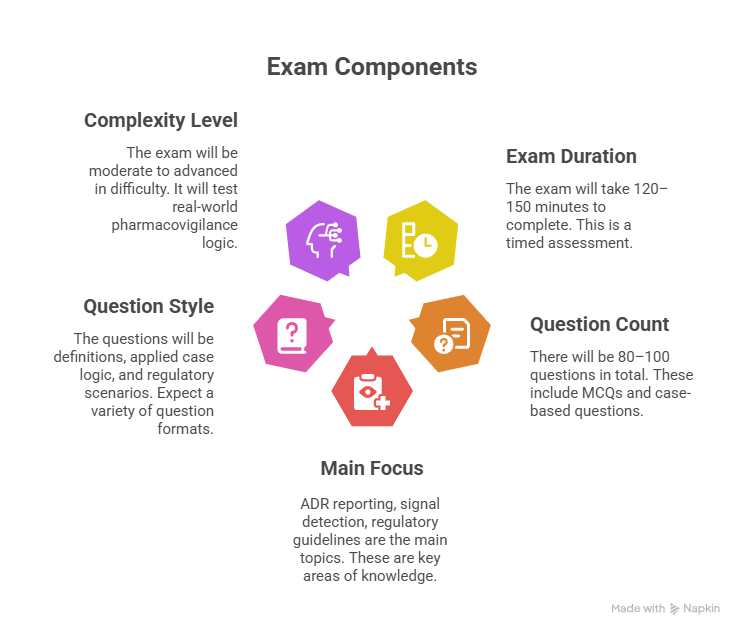
The pharmacovigilance certification exam is structured to reflect real-world safety operations, regulatory logic, and applied case management. Most exams span 120 to 150 minutes, with approximately 80 to 100 multiple-choice questions. Time is a factor—not due to volume, but due to the cognitive depth expected in scenario-based questions. The exam isn’t memorization-heavy; it tests interpretive thinking, regulation comprehension, and response strategy under pressure.
Expect domain breakdowns across:
Adverse Event (AE) and ADR Reporting – frequency, timelines, local vs. global requirements
Signal Detection and Risk Management – methods, tools (e.g., disproportionality analysis), and response
Regulatory Frameworks – FDA, EMA, ICH E2E, GVP Modules
Pharmacovigilance Systems Mastery – QPPVs, SOPs, quality audits
Data Management and MedDRA – hierarchy, coding, updates, application
Some certifications, like CCRPS’ Pharmacovigilance Certification, also integrate simulated case review and periodic knowledge checks across domains. This layered structure ensures you're not just recalling facts—you’re applying logic, policy, and critical clinical judgment.
Question Types: Case Analysis, Definitions, Regulatory Scenarios
The exam blends foundational definitions with deeply contextual case applications. Definitions focus on precise usage: know what distinguishes unexpected ADRs from serious ones, or how benefit-risk evaluation differs from signal detection. But memorizing terms won’t carry you far.
Case-based questions require you to:
Determine appropriate reporting timelines
Assess causality and rule out unrelated events
Navigate ethical conflicts in safety reporting
Choose suitable regulatory actions under differing regional laws
Regulatory scenarios test jurisdiction-specific decisions. For instance, you may be asked whether a US-based AE requires FDA MedWatch submission or qualifies under 21 CFR Part 314.80. You may also face EMA-focused questions on EudraVigilance reporting, or interpret ICH E2E guidelines for multinational post-market surveillance.
Expect multi-layered logic chains: one wrong assumption early in the case can lead to misjudged causality or improper documentation classification. This exam rewards clarity, not guesswork—strategic preparation is non-negotiable.
Key Areas of Focus in Exam Content
ADR Reporting, Signal Detection, Risk Management Plans
You cannot pass a pharmacovigilance certification exam without mastering the core trio: ADR reporting, signal detection, and risk management plans (RMPs). These are the pillars of real-world safety operations—and they show up heavily on the exam.
ADR Reporting: Know the precise definitions of serious, unexpected, and related ADRs. Understand timelines across regions: 15-day reporting for serious AEs under FDA and EMA, expedited vs. periodic submissions, and the difference between sponsor obligations and investigator responsibilities.
Source Recognition: Reports may originate from literature, spontaneous reports, patient-reported outcomes, or digital health systems. Recognizing valid reportable events is tested often.
Signal Detection: You must understand both qualitative (case clustering, clinical judgment) and quantitative (Bayesian, disproportionality) methods. MedDRA hierarchy knowledge is critical—especially how PTs roll up to SOCs in signal evaluation.
Risk Management Plans: Know what goes into an RMP—safety concerns, pharmacovigilance activities, and risk minimization measures. You’ll often be asked to assess whether a mitigation strategy (e.g., REMS) is proportional to the safety signal.
Scenario-based items might present incomplete case data and require a decision: Does this warrant expedited reporting? Is additional data required before risk minimization? These aren’t binary answers—they demand clinical safety judgment.
Regional Regulations: FDA, EMA, WHO, ICH
Pharmacovigilance is global. To succeed, you need to fluently compare and apply FDA, EMA, WHO, and ICH regulatory guidance. This portion of the exam frequently blends knowledge domains with scenario questions.
FDA: Familiarize yourself with 21 CFR Part 314.80, postmarketing surveillance (MedWatch Form FDA 3500A), and REMS program requirements.
EMA: Know Good Pharmacovigilance Practices (GVP) Modules, roles of the QPPV, and use of EudraVigilance and the EU-RMP format.
ICH: Deep dive into ICH E2E on pharmacovigilance planning and E2B(R3) on electronic reporting standards.
WHO: Understand the global harmonization role of Uppsala Monitoring Centre (UMC), the VigiBase database, and minimal data element requirements.
Expect questions that ask you to identify conflicting regulatory requirements and make the most compliant decision. You may be tested on reporting formats, submission methods, or prioritization of reporting when multiple authorities are involved.
Knowing the guidance documents isn’t enough—you must apply their intent. The exam measures your ability to act as a globally competent safety professional capable of protecting patients and managing compliance across jurisdictions.
Study Timeline Options for Working Professionals
4-Week and 8-Week Schedules
Whether you have a month or two, your pharmacovigilance exam study plan must be structured, adaptive, and ruthlessly focused. Most working professionals can’t afford 6-hour study days, so every minute counts. Below are two strategic paths:
4-Week Intensive Plan
This schedule demands 2–3 hours per weekday and 5–6 hours over weekends.
Week 1: Core theory—ADRs, MedDRA, signal detection
Week 2: Regulatory frameworks (FDA, EMA, ICH, WHO)
Week 3: RMPs, aggregate reports (PADERs, PSURs), safety databases
Week 4: Practice exams, review flagged weak points, focus on time efficiency
This works best for candidates with prior experience in drug safety, regulatory, or clinical research who need to reinforce and apply existing knowledge.
8-Week Balanced Plan
Designed for full-time professionals, requiring 1.5–2 hours daily and dedicated weekends.
Weeks 1–2: Foundations—key terms, ADR classifications, and data sources
Weeks 3–4: In-depth GVP, MedDRA application, causality assessment models
Weeks 5–6: Real-world practice—case study drills, mock timelines, documentation exercises
Weeks 7–8: Revision, flashcards, mixed-topic scenario testing
This longer runway gives space to master scenario-based questions and regulatory reasoning at a steady, deep-learning pace.
Flexible Review + Weekend Practice Testing
Regardless of your timeline, flexibility and reinforcement are essential. Working professionals must blend structured review with realistic practice under timed conditions. Build your study momentum through layered repetition:
Flashcard sprints (10–15 min blocks) to reinforce terminology and acronyms
Daily mini-quizzes (5–10 questions) to gauge retention
Case Study Saturday: Spend weekends simulating 2–3 real case questions, analyzing narrative structure, and identifying regulatory decisions
Sunday Recall Reviews: Recap the week’s material via notes, diagrams, or voice recordings
Use spaced repetition software (like Anki) to automate review frequency. Track weak areas weekly and adjust the next week’s focus accordingly.
Prioritize scenario comprehension over passive reading. If you have 30 minutes, invest it in applying principles—not skimming GVPs. This mindset builds the real-world skillset that both the exam and your future employer will expect.
| Plan Type | Suggested Duration & Strategy |
|---|---|
| 4-Week Intensive | 2–3 hrs/day, 5–6 hrs weekends; focus on simulation and regulatory drills |
| 8-Week Balanced | 1.5–2 hrs/day with weekend deep dives into GVP, case review, and timed practice |
| Flashcard Sessions | Daily 10–15 minute blocks to reinforce terminology and timelines |
| Weekend Practice Drills | Timed simulations and case deconstruction under real exam conditions |
| Spaced Repetition | Automated review cycles for long-term retention and memory activation |
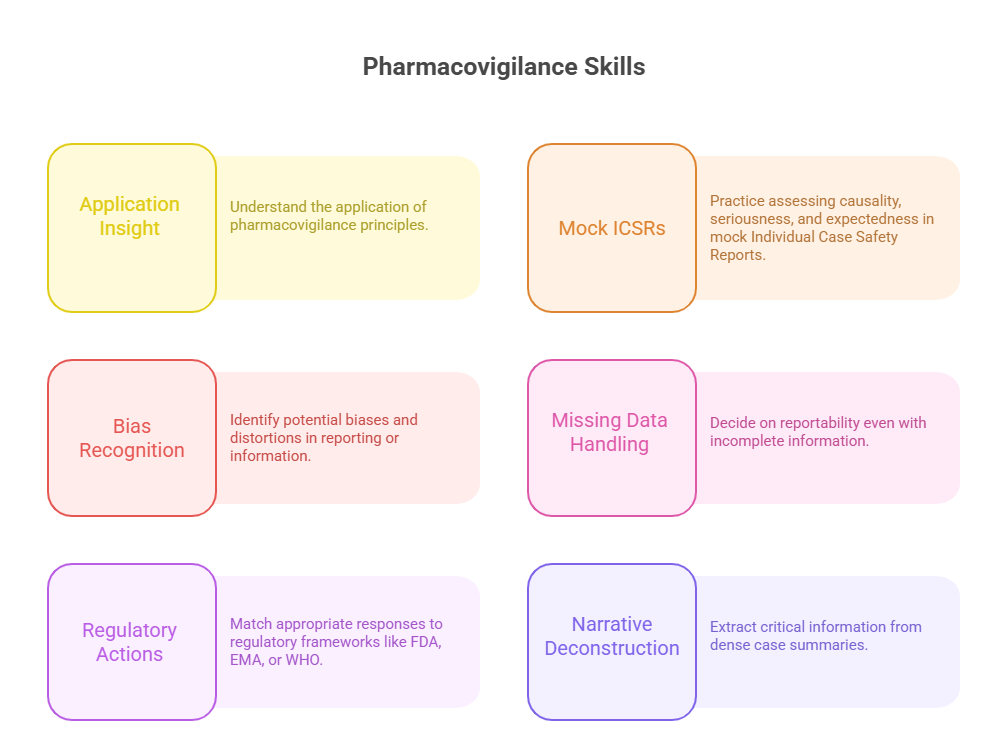
Mastering Case-Based and Scenario Questions
Reviewing Mock Case Reports
Case-based questions are the hardest part of the pharmacovigilance certification exam—and the most career-relevant. These items assess how well you apply theory under real-world pressure, not how many facts you’ve memorized. To succeed, you must practice interpreting mock case safety reports (ICSRs) like a trained PV specialist.
When reviewing mock cases:
Start by identifying core details: suspect drug, event(s), outcome, reporter type, and country of origin (for regional regulation).
Determine seriousness using ICH-E2D definitions: hospitalization, disability, congenital anomaly, or life-threatening events.
Evaluate expectedness using reference safety information (RSI), e.g., Investigator Brochure or Company Core Data Sheet.
Assess relatedness: Is there a plausible temporal association, dechallenge/rechallenge data, or alternative explanations?
Structure your answer using PV logic: event → drug → temporality → dechallenge → regulatory obligation. With enough repetition, this becomes second nature.
Many candidates fail by rushing to conclusions. Instead, slow down and walk through each ICSR line-by-line. Annotate key triggers, cross-reference with guideline expectations, and always ask: What would I report, and to whom?
Identifying Bias, Missing Data, and Causality Errors
The exam will test how you handle imperfect or incomplete reports. Expect questions with missing timelines, unclear outcomes, or conflicting data from reporters. The goal isn’t to “solve” the case—but to judge what action is most appropriate given what’s available.
Watch for:
Reporter Bias: Healthcare professionals may underreport subjective symptoms; patients may overstate causality. Identify how this affects signal reliability.
Missing Data: A lack of onset date, dosing info, or outcome status limits your causality judgment. Learn when it’s acceptable to report with incomplete data—and when follow-up is required.
Causality Pitfalls: Common traps include assuming temporal association = causality or ignoring polypharmacy. Always consider WHO-UMC or Naranjo algorithms to anchor your reasoning.
You may be asked to:
Choose between submitting a report now vs. following up for more data
Classify seriousness without final outcome confirmation
Distinguish a known side effect from a new safety signal based on frequency and severity
These questions favor exam-takers who think like safety reviewers, not just students. Build your reflexes by working through real or mock ICSRs and narrating your thought process aloud. The exam isn’t just testing correctness—it’s testing decision-making maturity.
Expert-Recommended Study Resources
WHO Handbooks, MedDRA, GVP Modules
Top scorers don’t study more—they study what actually appears on the pharmacovigilance certification exam. That means mastering regulatory handbooks, technical dictionaries, and structured pharmacovigilance guidance. These aren’t optional—they’re the exam’s blueprint.
Start with the WHO Pharmacovigilance Toolkit. It covers everything from minimum case criteria to signal prioritization and country-level reporting systems. Focus on modules around spontaneous reporting, causality assessment, and national pharmacovigilance centers.
Next: MedDRA. Learn the hierarchy—SOC, HLGT, HLT, PT, LLT—and practice coding mock adverse events. Don’t memorize terms—practice mapping clinical narratives to precise Preferred Terms (PTs) using MedDRA Browser.
Then, move to EMA’s GVP Modules (especially I to IX). These are gold. Study the roles of MAHs, QPPVs, and the requirements for PSURs, RMPs, and post-authorization safety studies. Print out Module VI (ADR collection and management) and annotate it—it’s heavily tested.
These sources train you to think like regulators, not just students. Mastery here directly boosts case analysis speed and accuracy.
High-Yield Flashcards and PV Databases
Beyond heavy texts, lean into efficient tools that help with fast recall and critical application.
Use flashcards for acronyms (e.g., REMS, RSI, CIOMS), MedDRA coding structures, and reporting timelines. Tools like Anki or Quizlet with spaced repetition are essential.
Create custom flashcards for case classification (serious vs. non-serious), causality factors, and database definitions (e.g., PADER vs. PSUR).
Integrate high-yield summary sheets with visual maps of risk management structures, ICH guidelines, and regulatory flowcharts.
For databases:
Familiarize yourself with VigiBase and FAERS layouts. Understand what each collects, their strengths/weaknesses, and how signal detection tools function.
Know how to extract relevant safety data for signal prioritization, including disproportionality analysis outputs and periodic trend graphs.
Also valuable: Recorded regulatory webinars from FDA, EMA, or WHO. These help translate dry regulations into real-life application scenarios, which are exactly what the exam targets.
Don't spread your energy thin. Stick to what exam setters actually pull from: WHO, EMA, MedDRA, ICH, and major PV databases. Combine strategic review with relentless scenario practice, and your prep will be both lean and lethal.
| Resource | Purpose/Benefit |
|---|---|
| WHO Pharmacovigilance Toolkit | Global PV framework, minimum reporting standards, safety terminology |
| MedDRA Browser | Term hierarchy practice, narrative-to-PT mapping, signal grouping |
| EMA GVP Modules I–IX | Guidance for EU PV systems, PSURs, RMPs, QPPV responsibilities |
| VigiBase & FAERS | Database structures, signal detection techniques, trend interpretation |
| Flashcards & Quizzes | Terminology recall, guideline comparison, exam-speed optimization |
How CCRPS’ Pharmacovigilance Certification Prepares You End-to-End
Covers 20+ Global Guidelines with Built-In Quizzes
The CCRPS Pharmacovigilance Certification is engineered for real-world safety roles—not academic theory. What sets it apart is its deep integration of 20+ international guidelines, presented in a format that mirrors how global pharmacovigilance teams actually operate.
You’ll study core frameworks including:
ICH E2E, E2B(R3), and E2D for risk planning and case handling
FDA 21 CFR Part 314.80 and REMS strategies
EMA GVP Modules I–IX, with case-based interpretations
WHO UMC safety protocols and minimum reporting standards
CIOMS VI–VIII for aggregate reporting and benefit-risk integration
Each module ends with interactive quizzes, ensuring high-yield learning is reinforced—not just skimmed. These aren’t generic MCQs—they reflect exam-level scenarios involving narrative analysis, causality decisions, and regulatory reporting choices.
Students can assess their grasp of regional laws, submission standards, and classification logic after each lesson—preparing not just for the certification exam, but for hands-on PV roles in clinical trials and post-market surveillance.
This isn’t passive learning. You’re constantly tested on how you’d act in a safety role, not just what you know.
Case Simulation Training + Lifetime Access
What truly distinguishes the CCRPS Pharmacovigilance Certification is its emphasis on case simulation. You don’t just read about ICSRs—you actively code them, assess their causality, and determine proper reporting channels. This simulation-driven model builds reflexes that match what global PV reviewers do daily.
You’ll practice real ADR scenarios: missing onset dates, conflicting reporter narratives, polypharmacy events
You’ll classify seriousness and relatedness, choose MedDRA terms, and simulate submission to EudraVigilance or MedWatch
You’ll be challenged with questions like: Would you expedite this report under FDA rules? Should it trigger an update to the RSI?
The simulations replicate FDA, EMA, and WHO environments, so you're constantly contextualizing your actions within regulatory systems. That means fewer surprises on exam day—and stronger job readiness after passing.
The program also includes lifetime access, meaning your certification isn’t a one-time cram. You’ll have ongoing access to updated modules, new regulatory releases, and fresh case studies—ensuring long-term professional utility.
In short: CCRPS isn’t just preparing you for a score. It prepares you to think, act, and perform like a safety leader, with tools built around real pharmacovigilance roles and global compliance expectations.
Frequently Asked Questions
-
The best way to prepare is to follow a focused, exam-aligned study plan that prioritizes regulatory frameworks, case-based questions, and signal detection principles. Start with WHO and ICH guidelines, then move into EMA GVP Modules and FDA reporting structures. Use flashcards for terminology, and schedule weekly case simulation reviews. Consider enrolling in a course like the CCRPS Pharmacovigilance Certification, which integrates interactive quizzes and real-world scenarios so you’re not just studying, but training like a working pharmacovigilance professional.
-
If you're balancing a full-time job, most professionals require 6 to 8 weeks of focused study. Aim for 1.5 to 2 hours daily on weekdays, and 4–6 hours on weekends. Prioritize high-impact topics like ADR reporting, MedDRA hierarchy, and regional regulatory obligations. Use spaced repetition to lock in terminology and simulate timed test sessions to build speed. Flexible courses like the CCRPS Pharmacovigilance Certification offer lifetime access, making it easier to pace your prep effectively around a work schedule.
-
Expect heavy emphasis on adverse event classification, signal detection methodology, risk management planning, and regulatory timelines. Many questions present incomplete case narratives, requiring decisions on seriousness, causality, and region-specific reporting. Mastering FDA's MedWatch, EMA’s EudraVigilance, and WHO’s minimal data elements is essential. You’ll also need to understand ICH E2E, MedDRA coding, and how to apply the GVP Modules in real-world pharmacovigilance workflows. Simulated case practice helps bridge theory and application—often the gap that separates pass from fail.
-
Yes—mock case scenarios are critical. They reflect how real safety reports are assessed, and most exam failures come from poor interpretation under pressure. Practicing mock ICSRs helps develop judgment in seriousness classification, regulatory urgency, and data completeness. You’ll also train your eye for biases, missing data, and multi-drug confounding. Programs like the CCRPS Pharmacovigilance Certification include built-in simulations with feedback, making them ideal for developing pattern recognition and decision speed—two things no textbook alone can teach.
-
The CCRPS Pharmacovigilance Certification stands out for its case simulation emphasis, lifetime access, and coverage of 20+ international guidelines. While many exams test definitions and isolated rules, CCRPS ensures you're mastering real-world application, with training modeled after global safety team workflows. It includes built-in quizzes, regulatory comparisons (FDA vs. EMA vs. WHO), and MedDRA coding practice. This makes it ideal for those seeking roles in clinical trials, pharma, CROs, or post-marketing surveillance environments where active decision-making is crucial.
-
Critical. MedDRA isn’t just a coding tool—it’s the backbone of signal detection, report classification, and data aggregation. You must understand the structure: System Organ Class (SOC) → High-Level Group Term (HLGT) → High-Level Term (HLT) → Preferred Term (PT) → Lowest Level Term (LLT). Expect questions where a clinical narrative must be matched to the correct PT or where you're asked to interpret signal strength across SOCs. Practicing MedDRA mapping directly improves both exam performance and real-world pharmacovigilance proficiency.
-
Yes, many exams include region-specific scenarios, particularly focused on FDA, EMA, ICH, and WHO guidelines. You might be asked to choose the right submission form (e.g., FDA MedWatch 3500A vs. EudraVigilance XML) or to decide which timeline applies (e.g., 7-day, 15-day, or 30-day reporting). Understanding differences like QPPV requirements under EMA or REMS programs in the U.S. is key. A certification like CCRPS covers all major frameworks and teaches candidates how to respond compliantly across multiple jurisdictions.
Final Thoughts
Passing the pharmacovigilance certification exam isn’t about memorizing endless regulations—it’s about mastering how to apply them under real-world pressure. Every scenario, question, and guideline you encounter is testing one thing: your ability to ensure patient safety through accurate, compliant, and timely decision-making.
From signal detection to global regulatory alignment, success on this exam requires strategic preparation, not passive reading. You must internalize frameworks like ICH E2E, act confidently in simulated case narratives, and identify risk with the same vigilance as a working PV officer. Whether you’re aiming to break into the field or elevate your drug safety career, this guide arms you with the tools to succeed.
| 📊 What’s your biggest challenge in preparing for the pharmacovigilance certification exam? | |
|---|---|
| Understanding complex regulatory frameworks (FDA, EMA, ICH, WHO) | |
| Applying MedDRA coding and interpreting case narratives | |
| Managing time and focus while working full-time | |