Passing Your Pharmacovigilance Exam Proven Preparation Strategies
Most candidates who fail the pharmacovigilance certification exam don’t do so because of a lack of effort—but because of the wrong kind of effort. Too many rely on passive reading, memorize outdated guides, or sprint through SOPs hoping it’ll all stick. But this exam isn’t about cramming—it’s about how well you think through real-world safety scenarios.
To succeed, you need a preparation strategy built on logic, case-based understanding, and regulatory reasoning. This guide offers exactly that. We’ve reverse-engineered what top scorers do differently, pulled in real test behaviors, and organized it into a system that anyone—regardless of background—can implement. If you’re serious about passing the pharmacovigilance exam—whether you’re a first-timer or retaker—this isn't just a guide. It’s your conversion roadmap.
Step-by-Step Study Framework That Works
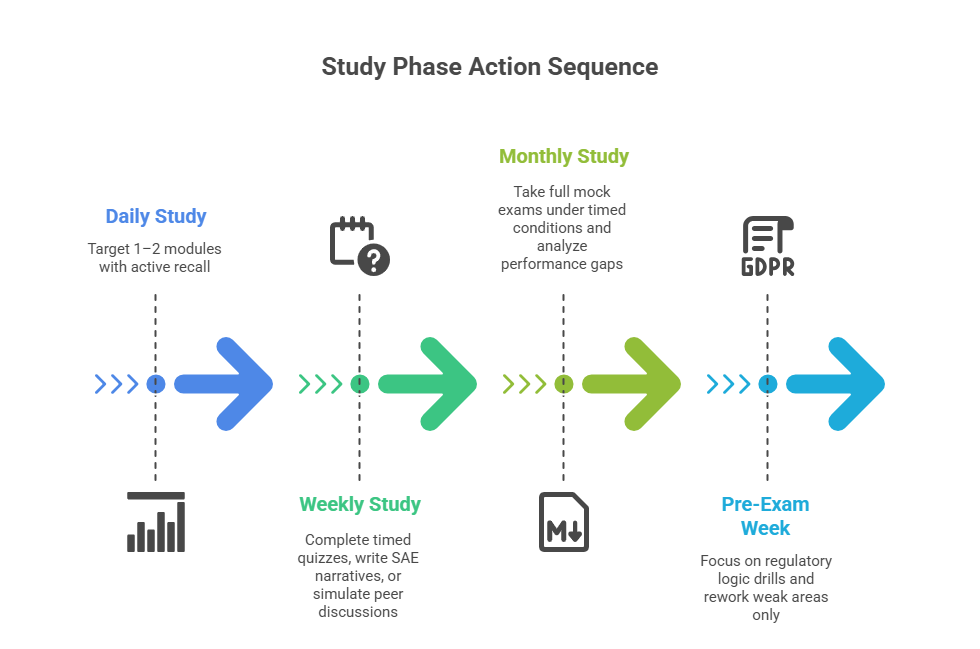
Most candidates underestimate how structured their pharmacovigilance study plan needs to be. Reading guidance documents sporadically or reviewing notes without benchmarks almost guarantees conceptual gaps that show up on exam day. A high-performance study framework needs to be broken into daily micro-goals, weekly knowledge checkpoints, and monthly simulation tests—with every session reinforcing case-based thinking.
Define Daily, Weekly, and Mock-Test Milestones
Daily Goals
Each study day must target specific modules (e.g., GVP Module VI or FDA FAERS reporting) with set time limits—typically 90–120 minutes. This isn’t passive reading. Use active recall: write out what each regulation means, how you’d apply it in an actual adverse event scenario, and what a case reviewer would expect to see in documentation.
Weekly Milestones
By the end of each week, validate your progress with one of three things:
A 25-question timed quiz
A self-written narrative of a hypothetical SAE
A peer-reviewed PV case discussion, if possible
These reinforce deep application and force you to synthesize rather than memorize.
Monthly Simulations
Simulate one full-length mock exam every 4 weeks. Replicate the real format: 3-hour timer, no breaks, and questions that challenge regulatory distinctions (e.g., differences in expedited reporting timelines between EMA and FDA). Use performance analytics to identify blind spots and revise weak areas before moving on.
Pair Reading with Application
Reading CIOMS guidelines, E2E pharmacovigilance standards, or ICH GCP documents means nothing unless you practice applying them. After reading any section, do one of the following:
Write out how you’d handle a real-world example based on it
Identify three possible case types where it would apply
Use the MedDRA browser to find appropriate PT/LLT codes based on that scenario
Every regulation must translate to patient case management or product safety logic in your preparation. If it doesn’t, you’re wasting your time.
What High-Scoring Candidates Do Differently
Top-performing candidates don’t just study harder—they study smarter by aligning every effort with the actual logic of pharmacovigilance assessments. They think in terms of risk minimization, causality assessment, and regulatory compliance, not just definitions or guidelines. Their preparation mirrors how a Qualified Person for Pharmacovigilance (QPPV) would reason through real reports, not how a student would cram.
Review Real Case Narratives and WHO Literature
The WHO’s VigiBase case narratives are goldmines for understanding how pharmacovigilance plays out in the real world. These aren't just theoretical scenarios—they show how spontaneous reports are compiled, evaluated, and submitted by professionals.
Here’s how top scorers use them:
They analyze reporting timelines, follow-up strategies, and signal outcomes.
They compare case handling across regions—what passes threshold in the EU may not trigger follow-up in the U.S.
They reverse-engineer what went wrong: What signal was missed? Was the narrative complete? Were expected vs. unexpected events coded correctly?
In parallel, they study WHO-UMC causality assessment criteria to understand global logic used in signal validation—especially how regulators balance temporal relationship vs. biological plausibility.
Use MedDRA Browser + EU Module Templates
The MedDRA browser isn’t just a lookup tool—it’s a core exam prep resource. High scorers don’t just memorize terms. They understand how PTs (Preferred Terms) roll up to HLTs and SOCs, and they regularly practice assigning accurate terms to fictional narratives.
They also lean heavily on EU GVP Module templates—especially for:
Module VI (Management of ADRs)
Module IX (Signal Management)
Module VII (Periodic Safety Update Reports)
They create mock PBRERs, fill in signal detection flowcharts, and treat their study sessions like mini pharmacovigilance work simulations.
This mindset—studying as if you're already a PV professional—is what separates the top 10% from everyone else.
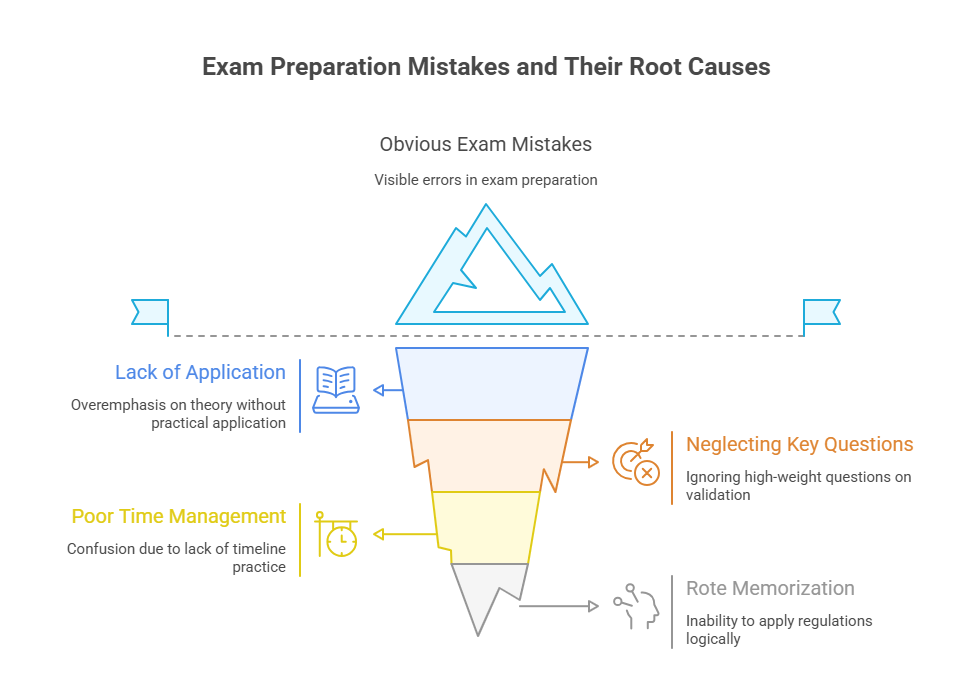
Avoid These Exam Prep Pitfalls
Even the most dedicated pharmacovigilance exam candidates can fail from using the wrong preparation methods. The issue isn’t effort—it’s inefficiency. Certain traps derail your learning curve, waste study hours, and leave you underprepared for scenario-based questions. Spotting and avoiding these early gives you a strategic edge.
Overloading on Textbooks
Pharmacovigilance isn’t an academic subject—it’s a regulatory function. Candidates who hoard textbooks often drown in theory and fail to bridge knowledge to practice. Reading 700 pages of pharmacology or adverse event classification won't help you if you can’t answer a question like:
"What expedited timeline applies to a SUSAR occurring during a blinded global trial with dual regulatory oversight?"
Instead, use this rule: for every hour of reading, spend 30–45 minutes applying it through either MCQs, mock narratives, or regulatory flowchart mapping. Tools like GVP module PDF highlighters, CIOMS I report walkthroughs, and case drills give better ROI than general pharmacovigilance encyclopedias.
Skipping Signal Detection Scenarios
One of the biggest gaps in failed attempts is ignoring signal detection and validation. Many candidates focus on spontaneous report handling but forget the exam expects you to:
Differentiate between a potential signal, validated signal, and refuted signal
Understand how disproportionality analysis leads to downstream safety actions
Use algorithms like PRR, ROR, or Bayesian confidence propagation neural networks
And most importantly—how to explain these decisions in a concise written format within time pressure. These aren't optional topics—they are core decision-making areas that show up as case-based MCQs.
If your prep doesn’t include signal tracking exercises using public datasets or you’ve never manually filled out a signal detection log, your strategy needs an overhaul.
Practice-Driven Tools That Accelerate Learning
Reading guidelines and frameworks is foundational—but it’s practice-based learning that drives mastery. What separates a confident exam-taker from a panicked one is repetition in simulated environments using real-world tools. These aren’t just supplements—they’re core components of any efficient pharmacovigilance exam prep strategy.
Interactive Flashcards, Mock ADR Reports, Quizzes
Digital flashcards built around MedDRA hierarchy, GVP modules, and ICSR fields work better than re-reading notes. Use spaced repetition apps like Anki or Brainscape to repeatedly drill:
Key submission timelines for SUSARs and PSURs
Decision trees in causality assessment
Differences in expedited reporting between EMA and FDA
But don’t stop at memorization. Mock ADR reports are where serious gains happen. Practice drafting adverse event reports from fictional case narratives. Include elements like:
Onset dates vs. administration dates
Concomitant medication tables
WHO-UMC causality reasoning
Then score yourself using templates from CIOMS or ICH E2B(R3) to pinpoint structural or content weaknesses.
Supplement that with timed quizzes—minimum 25 questions per topic. Use randomized question banks that simulate exam wording and structure.
PV Software Simulations and SOP Templates
To excel in the exam’s case-based questions, you must simulate what pharmacovigilance professionals actually do. Start with safety database tools or demos like:
Oracle Argus Safety
Veeva Vault Safety
PV-Works
Even if you only use screenshots or walkthroughs, study how data entry, narrative building, coding, and submission workflows operate. Pay attention to automation flags, reconciliation features, and audit trails.
Next, work with standard operating procedure (SOP) templates from public sources or mock companies. Rewriting or annotating SOPs for SAE handling, signal validation, or PSMF updates builds functional fluency in how pharmacovigilance teams run globally compliant operations.
This kind of immersive practice—from keyboard to case logic—is how top candidates lock in real recall under pressure.
| Tool | How to Use It |
|---|---|
| Flashcards | Create flashcards for regulatory timelines, MedDRA coding, and causality rules using spaced repetition apps like Anki or Brainscape. |
| Mock ADR Reports | Write sample adverse event narratives and populate CIOMS forms. Focus on timelines, case structure, and consistency in MedDRA terms. |
| Timed Quizzes | Take 25–50 question quizzes on signal detection, expedited reporting, and GVP scenarios under time constraints. |
| PV Software Simulations | Use tutorials or demo accounts of Argus, Veeva, or PV-Works to simulate real safety database workflows and case data entry. |
Creating a Test-Day Strategy
Passing the pharmacovigilance exam isn’t just about what you know—it’s about how you use that knowledge under pressure. Candidates often walk in well-prepared but still underperform due to poor time allocation, second-guessing, or inefficient elimination strategies. A precise test-day approach converts months of study into a high-scoring performance.
Eliminate Choices Using Regulatory Logic
When faced with close-call multiple-choice questions, never rely on gut instinct. Use regulatory exclusion logic to eliminate options methodically:
Look for jurisdiction mismatches. If a question references a PSUR but gives FDA timelines, eliminate it. FDA uses PADERs, not PSURs.
Discard answers with vague terminology. Regulators never say “as soon as possible”—they assign specific timelines (e.g., 7 or 15 days).
Use cause-effect logic. If a SUSAR occurs in a blinded trial and unblinding is required, ask: does this affect reporting timelines under EMA’s GVP Module VI?
These methods allow you to remove at least two options in most MCQs, especially those testing timeline accuracy, report classification, or role delegation (e.g., MAH vs. QPPV responsibilities).
Time Management for 3-Hour Exams
A 180-minute exam with 100+ scenario-based questions means you have less than 2 minutes per item. Candidates who don’t segment their time typically panic after the halfway mark. Use this time framework:
First 90 minutes: Aim to complete 60–65 questions—flag the ambiguous ones but don’t stop.
Next 60 minutes: Return to flagged items. Revisit questions requiring literature logic, like signal validation or narrative evaluation.
Final 30 minutes: Perform a logic sweep—double-check that every answer aligns with regulatory source principles, not just memory.
Have a pre-logged answer review strategy: skim the bolded terms in each question, and re-scan marked ones using rule-based logic.
Your mindset on test day should be: “If I were a regulator, how would I justify this answer in a pharmacovigilance audit?”
| Component | Strategy |
|---|---|
| MCQ Elimination | Use regulatory logic to eliminate options—focus on jurisdiction mismatches, vague language, or non-compliant timelines. |
| Time Blocks | Break exam into three segments: first 90 mins for fast pass, next 60 mins to revisit flagged questions, final 30 mins for logic sweep. |
| Flagged Questions | Mark tough questions and return with fresh logic—reassess using guideline timelines and causality rules. |
| Mindset | Think like a QPPV or regulator. Ask: “What’s the most compliant, audit-proof answer here?” |
Why CCRPS’ PV Certification Course Is Built for Exam Success
Most exam prep programs fail because they only teach what to memorize. The CCRPS Pharmacovigilance Certification is built differently—it trains you to think like a regulatory professional, not a test-taker. Every module, every tool, and every update is engineered around real-world PV logic, so you walk into the exam already fluent in how global systems work.
Case-Based + Compliance-Focused Modules
Each section of the CCRPS PV Certification uses actual case-based scenarios drawn from global regulatory submissions. You're not just learning theory—you’re solving problems:
Drafting narrative reports for suspected unexpected serious adverse reactions (SUSARs)
Completing mock CIOMS I forms based on simulated drug exposure timelines
Using coding logic to assign appropriate MedDRA terms from raw physician notes
Unlike generic courses, CCRPS trains you to make judgment calls under uncertainty—the exact skill the exam evaluates. Modules also break down GVP requirements, FDA FAERS workflows, and WHO causality criteria, showing you how they compare and when each applies.
Access to Updates on Global PV Regulations
Pharmacovigilance regulations evolve constantly. What was compliant two years ago may now violate EMA or FDA expectations. CCRPS provides lifetime access to updates, including:
Changes in E2B(R3) reporting structures
Adjustments to DSUR/PSUR requirements
Revised EU GVP modules and ICH guideline shifts
You also receive early-access briefings on global signal detection strategies, digital PV tools, and AI-driven narrative processing—critical for staying ahead of curveball exam questions.
Most importantly, the course includes full-length mock exams, graded narrative assignments, and access to regulatory experts who provide direct feedback—mirroring the complexity and structure of the real exam.
If you’re targeting exam success with precision—not guesswork—this certification gives you the structured path and tools to get there.
Frequently Asked Questions
-
The best starting point is to align your prep around real-world pharmacovigilance tasks—not just theoretical reading. Begin by reviewing global regulations like ICH E2E, CIOMS guidelines, and GVP Modules I–IX. As you study each section, create short case scenarios and apply the rules actively. Use tools like the MedDRA browser, CIOMS templates, and mock ICSR forms to mimic professional workflows. Your first two weeks should focus on mastering terminology, understanding ADR classifications, and building a study calendar that incorporates weekly quizzes, monthly mock exams, and practice narratives.
-
Most successful candidates allocate 8–12 hours per week for at least 8 to 10 weeks, depending on prior experience. However, quality trumps quantity. You need structured time blocks: 2–3 sessions per week for reading, 1 for mock testing, and 1 dedicated to writing mock narratives or reviewing real case studies. As the exam nears, increase focus on timed drills and compliance decision-making exercises. Avoid studying in fragmented intervals—sustained attention during 90–120 minute deep work sessions improves retention and mirrors test-day endurance.
-
High-weight areas include adverse event classification, expedited reporting timelines, causality assessment, and global regulatory differences. Expect deep questions on SUSAR criteria, PBRER structure, signal validation processes, and QPPV responsibilities under EU GVP. Case-based questions will test your ability to apply ICH E2B guidelines, interpret source data, and select the correct regulatory path. Also prepare for MedDRA hierarchy usage, data reconciliation, and narrative completeness. To score high, treat your prep as if you're managing a live PV case—not just answering a test.
-
No, prior pharmacovigilance experience is not required—but understanding how regulators evaluate cases is essential. The exam is structured to assess how well you simulate critical thinking under regulatory constraints, not whether you’ve worked in a CRO or pharma company. With the right resources—especially case-based prep like that in the CCRPS Pharmacovigilance Certification—you can bridge the gap. Use SOP templates, mock ICSRs, and global safety databases to build familiarity with workflows. This approach levels the field even if you’re new to the domain.
-
Use regulatory comparison matrices and flowcharts to visually map submission deadlines by region and case type. Practice converting scenarios into decisions—e.g., if a serious unexpected reaction occurs during a Phase III EU trial, when is it due? Memorize anchor timelines: 7 or 15 days for SUSARs, 30–90 days for PSURs, and regional exceptions (like FDA’s PADER format). Integrate this with daily flashcard drills and mock case walkthroughs. Understanding the logic behind why a report is due when it is makes retention easier and boosts speed under exam pressure.
-
Practicing with the MedDRA browser is non-negotiable. The exam doesn’t just test if you know what MedDRA is—it tests your ability to apply it accurately to diverse adverse events. You must understand how to navigate from LLT to PT to SOC, distinguish similar terms, and ensure consistent terminology usage across data points. Practice coding fictional AEs into the correct hierarchy. Top candidates often challenge themselves by backcoding: given a PT, can they recreate the AE narrative that generated it? This reverse engineering locks in fluency
-
No. You should internalize the structure, logic, and application of these guidelines—not memorize them verbatim. Focus on their use in case assessment, signal detection, and reporting standards. For example, understand the structure of a CIOMS I form, the essential components of ICH E2E risk management planning, and when to apply E2B(R3) format changes. Break down each guideline into scenario types: What happens when a product triggers a signal in Asia vs. Europe? Mastering how these guidelines guide action is more important than rote recall.
Final Thoughts
Passing your pharmacovigilance exam isn’t about how many hours you study—it’s about how deeply you engage with case-based logic. Candidates who treat prep like a checklist often miss the point: this exam assesses your ability to apply regulatory knowledge in real-world safety decisions, not recite facts.
Every successful candidate invests in mastering regulatory frameworks, practicing with tools like MedDRA and CIOMS, and simulating actual PV workflows. They don’t just read—they write narratives, time their answers, and think like safety reviewers.
If you’ve been studying hard but still feel unsure, shift your focus from memorization to structured simulation and pattern recognition. That’s where performance leaps happen.
| 📊 Quick Poll: What’s Your Biggest Challenge in PV Exam Prep? | |
|---|---|