Pharmacovigilance Specialist Salaries and Career Growth 2025 Industry Report
The Pharmacovigilance Specialist role is more critical than ever in 2025, as global regulators and pharmaceutical companies increase oversight on drug safety. Pharmacovigilance (PV) professionals are responsible for monitoring, assessing, and preventing adverse drug reactions, making their expertise indispensable throughout the drug development lifecycle. As clinical trials expand and post-marketing surveillance becomes more complex, demand for qualified specialists is rising rapidly, driving significant salary growth worldwide.
Beyond compensation, this analysis explores career growth opportunities in pharmacovigilance, including advancement to leadership roles such as PV Manager, Drug Safety Officer, or Director of Pharmacovigilance. It also highlights how certifications, especially the CCRPS Pharmacovigilance Certification, can accelerate salary growth by validating skills in global safety reporting, regulatory compliance, and advanced signal detection. Whether you’re entering the field or advancing your PV career, this 2025 salary report provides the insights needed to benchmark compensation and plan for the future.
The Role of a Pharmacovigilance Specialist
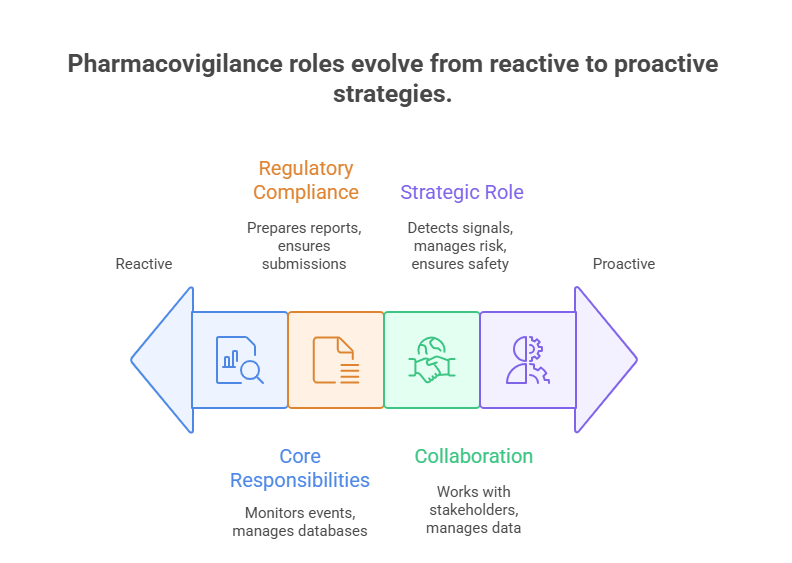
Core Responsibilities
The Pharmacovigilance Specialist (PV Specialist) is responsible for monitoring drug safety during clinical trials and post-marketing surveillance. They collect, analyze, and report adverse drug reactions (ADRs) to ensure compliance with regulatory requirements such as FDA, EMA, and ICH guidelines. In 2025, PV specialists play an increasingly digital role, using safety databases and signal detection software to identify potential risks quickly. Their oversight ensures both patient safety and the credibility of pharmaceutical companies.
Regulatory Compliance and Reporting
PV Specialists serve as the primary link between pharmaceutical companies and regulatory agencies. They prepare Individual Case Safety Reports (ICSRs), Periodic Safety Update Reports (PSURs), and Development Safety Update Reports (DSURs). Accuracy and timeliness are critical, as regulatory authorities can reject trial data if reporting standards are not met. With regulators tightening oversight globally, PV specialists are essential for ensuring Good Pharmacovigilance Practices (GVP), reducing compliance risks, and supporting successful drug approvals.
Collaboration Across Functions
Pharmacovigilance Specialists work closely with cross-functional teams, including clinical research associates, medical monitors, regulatory affairs specialists, and data managers. They provide safety insights during trial design, help investigators assess adverse events, and guide regulatory strategy. In post-marketing settings, PV specialists monitor spontaneous reports, literature, and real-world data for safety signals. Their collaboration ensures trial continuity and regulatory success, making them an integral part of the clinical research ecosystem.
Evolving Role in 2025
In 2025, the PV Specialist role is expanding due to the complexity of biologics, gene therapies, and oncology drugs. Specialists are now expected to use artificial intelligence tools for signal detection, manage global pharmacovigilance networks, and contribute to risk management plans. Their responsibilities extend beyond compliance to include strategic safety decision-making. This evolution makes the PV Specialist not only a compliance enforcer but also a strategic contributor to clinical development and patient safety worldwide.
Average Salaries by Region
USA & Canada
In 2025, Pharmacovigilance Specialists in the United States earn between $85,000–$105,000 annually, with senior professionals exceeding $120,000 in high-demand areas like oncology and biologics. Major hubs such as Boston, San Francisco, and New Jersey offer salaries at the top of this range due to their concentration of pharmaceutical companies. Entry-level PV specialists typically start at $65,000–$75,000, but certifications and advanced degrees accelerate growth. In Canada, average salaries range from CAD 75,000–95,000, with senior roles surpassing CAD 110,000. Benefits packages in Canada, such as healthcare and pension contributions, often balance slightly lower salaries compared to the U.S. Overall, North America remains one of the most lucrative regions for pharmacovigilance careers.
Demand is especially strong for PV specialists with CCRPS Pharmacovigilance Certification, as employers increasingly seek credentialed professionals who can reduce compliance risks. Companies are also offering relocation incentives and remote work flexibility to attract and retain top talent. The North American market shows steady salary growth of 5–7% annually, fueled by a shortage of experienced professionals and the rapid expansion of post-marketing surveillance requirements.
Europe
European Pharmacovigilance Specialist salaries vary widely by country. In the United Kingdom, PV specialists earn £45,000–£60,000, with senior roles reaching £75,000+. Germany offers average salaries of €55,000–€70,000, with top-tier professionals surpassing €85,000. Switzerland leads Europe, paying between CHF 90,000–110,000, often exceeding CHF 120,000 for senior positions. France, Spain, and Italy average slightly lower salaries, around €45,000–€65,000, though demand is rising in oncology and rare disease trials.
Language skills are a significant factor in Europe, with multilingual PV specialists often earning 10–15% more due to cross-border trial management. Regulatory expertise also influences pay, particularly for those with EMA safety reporting experience. Post-Brexit, the UK has seen companies increase salaries and offer retention bonuses to maintain talent. Switzerland continues to attract professionals across Europe due to its strong pharma sector and higher pay scales. Europe’s diversity makes regional benchmarking essential for both job seekers and employers.
Asia-Pacific
The Asia-Pacific region is showing the fastest growth in pharmacovigilance salaries. In India, PV specialists earn between $20,000–$35,000, with senior roles reaching $45,000. Although lower in absolute terms, annual growth rates exceed 12–15%, making India a hotbed for outsourcing pharmacovigilance activities. China offers higher salaries, averaging $35,000–$50,000, with top roles exceeding $60,000 at multinational CROs. Singapore leads the region, with PV salaries of $55,000–$75,000, aligning closer to Western benchmarks. Australia is another high-paying market, averaging AUD 90,000–110,000.
Employers across Asia-Pacific are prioritizing certification and digital safety skills, paying premiums for professionals with international experience. Multinational sponsors increasingly standardize pay scales, narrowing the gap between emerging and mature markets. This region provides exceptional career acceleration for young professionals, as rapid trial expansion in oncology and vaccines creates an ongoing demand for pharmacovigilance expertise.
Middle East
In the Middle East, pharmacovigilance salaries are rising steadily as Gulf nations expand healthcare research infrastructure. In the UAE and Saudi Arabia, PV specialists earn $50,000–$70,000 annually, often supplemented with tax-free income, housing, and relocation allowances. Senior roles in global pharma subsidiaries exceed $80,000, making the region competitive with Europe. Qatar and Kuwait also offer attractive packages, though opportunities are fewer compared to the UAE and Saudi Arabia.
Africa presents a mixed picture, with South Africa leading at $30,000–$45,000, while other African nations remain under $25,000 due to limited research infrastructure. However, global sponsors are outsourcing pharmacovigilance to African hubs, driving gradual salary growth. Bilingual and regulatory-trained PV specialists in these regions are highly valued, earning up to 15% more than peers. The Middle East offers strong compensation growth, particularly for certified professionals willing to relocate, with salaries expected to rise by 10% annually through 2030.
| Region | Average Salary (2025) | Senior-Level Salary | Notes |
|---|---|---|---|
| USA & Canada | $85,000 – $105,000 (U.S.) CAD 75,000 – 95,000 (Canada) |
$120,000+ | Highest global benchmark; strong benefits in Canada |
| Europe | £45,000 – £60,000 (UK) €55,000 – €70,000 (Germany) CHF 90,000 – 110,000 (Switzerland) |
£75,000+ / €85,000+ / CHF 120,000+ | Switzerland leads; multilingual staff earn premiums |
| Asia-Pacific | $20,000 – $35,000 (India) $35,000 – $50,000 (China) $55,000 – $75,000 (Singapore) AUD 90,000 – 110,000 (Australia) |
$45,000+ / $60,000+ / $90,000+ / AUD 120,000+ | Fastest YOY growth; CROs drive salary increases |
| Middle East & Africa | $50,000 – $70,000 (UAE, Saudi) $30,000 – $45,000 (South Africa) |
$80,000+ | Tax-free benefits and relocation packages common |
Salary Influencing Factors
Education & Certification
Educational background and certification are major drivers of pharmacovigilance salaries. While a bachelor’s degree in life sciences provides entry, advanced degrees or certifications increase earning potential. The CCRPS Pharmacovigilance Certification is one of the strongest differentiators in 2025, boosting pay by 10–20% globally. Employers value certified professionals because they demonstrate advanced knowledge of global safety reporting, signal detection, and regulatory compliance. In emerging regions, certification often closes the gap with Western salary benchmarks.
Experience & Seniority
Experience remains the single biggest salary driver. Entry-level PV specialists typically earn lower ranges ($20,000–$35,000 in India or £45,000 in the UK), but after 5+ years, salaries increase sharply. Senior PV specialists in North America can exceed $120,000 annually, reflecting their expertise in managing complex safety databases and regulatory submissions. Professionals who have led safety teams or supported global product launches are especially well-compensated. Employers increasingly offer retention bonuses to experienced staff to prevent turnover, further raising total compensation.
Employer Type & Therapeutic Area
Employer type significantly impacts salaries. Pharmaceutical companies and multinational CROs generally pay more than academic institutions or local research organizations. Specialists working in oncology, gene therapy, or rare disease trials earn 15–25% higher salaries because of the complexity and regulatory scrutiny of these therapies. In 2025, demand for PV specialists with expertise in biologics is particularly strong, as global regulators emphasize risk management plans for advanced therapies.
Geography & Cost of Living
Geographic location continues to influence base salaries. North America and Switzerland pay the highest global wages, while Asia-Pacific and the Middle East are experiencing the fastest salary growth. In lower-cost regions like India, salaries are rising 12–15% annually, quickly improving global competitiveness. Employers in high-demand regions often provide relocation allowances, housing benefits, or tax-free packages to attract talent. For professionals seeking global careers, mobility across regions remains a powerful way to increase long-term compensation.
Long-Term Career Growth in Pharmacovigilance
Entry-Level to Mid-Level Progression
Most professionals begin as Pharmacovigilance Associates or Specialists, focusing on case processing, safety database entry, and regulatory submissions. Salaries start modestly but grow steadily within 2–3 years as skills in adverse event reporting and global safety standards develop. By mid-level, specialists move into signal detection and risk management roles, often earning 20–30% more than at entry-level. Certification accelerates this transition, positioning candidates for higher responsibility roles earlier in their careers.
Advancing to Pharmacovigilance Manager
After 5–7 years of experience, many specialists advance into Pharmacovigilance Manager roles. Managers oversee safety teams, lead case reviews, and coordinate global reporting systems. In North America and Europe, PV Managers typically earn $120,000–$140,000 annually, while in Asia-Pacific salaries average $70,000–$90,000. Managers also play a strategic role, guiding risk management plans and interactions with regulatory agencies. Leadership, team management, and advanced regulatory knowledge are key drivers of career growth at this level.
Senior Roles and Global Leadership
The next career stage is Senior Pharmacovigilance Manager, Safety Officer, or Director of Pharmacovigilance. These roles involve oversight of global safety operations, regulatory strategy, and product portfolio safety. Salaries often exceed $150,000–$180,000 in North America, with Europe and Asia-Pacific offering slightly lower but competitive packages. Directors and Global Heads of PV also shape company policies, interact with international regulatory bodies, and prepare for product launches. Professionals at this stage frequently secure cross-border assignments, further increasing their total compensation.
Career Diversification Opportunities
Pharmacovigilance careers also provide opportunities for diversification. Experienced professionals often transition into regulatory affairs, medical affairs, or drug development leadership. Others specialize in safety data science, using AI-driven tools for predictive safety modeling—a growing niche with strong pay potential. Some PV specialists move into consultancy, offering expertise to CROs and biotech startups. These career paths diversify income streams and provide long-term stability, particularly for those with strong certifications and global regulatory experience.
The Role of Certification in Long-Term Growth
The CCRPS Pharmacovigilance Certification remains a vital accelerator for long-term career success. Certified professionals achieve promotions faster, secure higher salaries, and gain access to international roles. Employers consistently prioritize certification when hiring for senior positions, as it demonstrates mastery of safety reporting and regulatory compliance. Over the course of a career, certification can add hundreds of thousands in cumulative earnings, making it a strategic investment for any PV professional seeking leadership roles.
| Career Stage | Average Salary (2025) | Responsibilities | Notes |
|---|---|---|---|
| Entry-Level (Associate) | $40,000 – $60,000 (U.S.) | Case processing, ADR reporting, database entry | Certification accelerates hiring |
| Mid-Level Specialist | $70,000 – $90,000 | Signal detection, risk management, compliance oversight | Salary grows 20–30% over entry level |
| Pharmacovigilance Manager | $120,000 – $140,000 (U.S.) | Team leadership, global safety reporting, audits | High demand in oncology & biologics |
| Director of PV | $150,000 – $180,000+ | Global safety strategy, regulatory authority liaison | Cross-border mobility adds salary benefits |
Industry Trends Impacting Pay in 2025
Expansion of Biologics and Gene Therapies
One of the strongest trends driving pharmacovigilance salaries in 2025 is the rapid growth of biologics, cell, and gene therapies. These complex treatments demand intensive safety monitoring due to higher risk profiles. PV specialists with expertise in these therapeutic areas command 15–25% higher salaries than peers in general drug development. As more advanced therapies enter late-stage pipelines, the need for skilled pharmacovigilance professionals is expected to rise sharply.
Increased Global Regulatory Oversight
Regulatory agencies worldwide are tightening Good Pharmacovigilance Practices (GVP) standards. The FDA, EMA, and emerging authorities in Asia and the Middle East are enforcing stricter safety reporting requirements. PV specialists who can navigate multinational regulations and manage large volumes of case data are seeing salary premiums. In 2025, demand is especially high for professionals skilled in risk management planning and expedited reporting, as compliance failures carry major financial risks for sponsors.
Digital Transformation of Safety Monitoring
The shift toward AI-driven safety systems and real-world evidence (RWE) analysis is transforming pharmacovigilance. Specialists who can work with safety databases, predictive analytics, and automated signal detection tools are in short supply. Employers pay premiums for digital-ready PV specialists, particularly in global CROs and large pharma companies. In many regions, digital skills add 10–15% to base salaries, reflecting the strategic importance of data-driven pharmacovigilance in modern drug development.
Outsourcing and Emerging Market Growth
Pharmacovigilance outsourcing continues to expand, especially in India, China, and the Middle East, where CROs are building large safety operations. Salaries in these regions are rising quickly as global sponsors standardize pay closer to Western benchmarks. Relocation packages and remote roles further enhance total compensation. For certified PV specialists, outsourcing hubs present strong career growth opportunities, offering international exposure and rapid salary progression.
How APRAC Certification Accelerates Salaries and Career Growth
Global Recognition and Salary Impact
The Advanced International Pharmacovigilance and Regulatory Affairs Certification (APRAC) has become a gold standard credential for safety professionals in 2025. Employers worldwide recognize APRAC as proof of advanced expertise in global safety reporting, risk management planning, and regulatory affairs. Certified professionals consistently earn 15–25% higher salaries compared to non-certified peers. For entry-level specialists, APRAC accelerates hiring and boosts starting pay, while for experienced professionals, it strengthens leverage for promotions into senior roles.
Career Progression and Leadership Roles
APRAC certification significantly accelerates progression to Pharmacovigilance Manager, Safety Officer, and Regulatory Affairs Director roles. These positions often command salaries above $140,000–$180,000 annually in mature markets. Certified professionals are trusted to lead global safety operations, interact with agencies like the FDA and EMA, and manage risk mitigation strategies for biologics and advanced therapies. APRAC graduates are frequently shortlisted for leadership roles due to their validated expertise in both pharmacovigilance and regulatory affairs.
International Mobility and Cross-Border Advantage
The international focus of APRAC makes it especially valuable in today’s globalized clinical research landscape. Certified professionals can more easily transition between markets in North America, Europe, Asia-Pacific, and the Middle East, as employers recognize APRAC as a standardized credential. This global portability often translates into relocation packages, tax-free salary benefits, and international project leadership opportunities. In outsourcing hubs like India and the UAE, APRAC-certified specialists command salaries closer to Western benchmarks.
Long-Term Career Security
The demand for skilled pharmacovigilance and regulatory professionals is rising as clinical pipelines expand into complex therapies. APRAC certification provides long-term career security by ensuring professionals remain competitive in an evolving job market. Certified specialists advance faster, earn more, and gain access to senior-level decision-making positions. Over a career span, APRAC can add hundreds of thousands of dollars in cumulative salary growth, making it one of the most impactful investments for pharmacovigilance and regulatory professionals worldwide.
Frequently Asked Questions
-
The average salary for a Pharmacovigilance Specialist in 2025 varies significantly by region. In the U.S., salaries range from $85,000–$105,000, with senior roles exceeding $120,000. In Canada, the average is CAD 75,000–95,000. European salaries differ widely: the UK averages £45,000–£60,000, Germany €55,000–€70,000, and Switzerland leads with CHF 90,000–110,000. In Asia-Pacific, India averages $20,000–$35,000, China $35,000–$50,000, and Singapore $55,000–$75,000. The Middle East pays $50,000–$70,000, often tax-free. Salaries are rising globally, driven by expanding trial activity, stricter regulatory requirements, and the complexity of biologics and gene therapies.
-
In 2025, the highest-paying countries for pharmacovigilance specialists are the U.S., Switzerland, and Singapore. U.S. professionals average $85,000–$105,000, with senior positions surpassing $120,000. Switzerland offers CHF 90,000–110,000, setting the European benchmark. Singapore averages $55,000–$75,000, aligning closer to Western standards due to strong government support for research and clinical development. Germany and Canada also pay competitively, though slightly lower. By contrast, India and Brazil offer lower absolute salaries but have double-digit annual growth rates, narrowing the global gap. Specialists with certifications like APRAC (Advanced International Pharmacovigilance and Regulatory Affairs Certification) consistently earn more across all regions.
-
Certification is one of the clearest salary boosters in pharmacovigilance. In 2025, professionals with the APRAC certification earn 15–25% more than non-certified peers. For entry-level roles, this translates into a starting salary advantage of $8,000–$10,000 annually in North America and Europe. In emerging markets, certification is often the deciding factor for hiring into multinational CROs, where salaries align closer to Western benchmarks. Beyond base pay, certification strengthens promotion prospects, leading to faster career progression into management roles. Over a 10–15 year career, certified specialists can earn hundreds of thousands more cumulatively compared to their non-certified counterparts.
-
Yes, pharmacovigilance salaries vary widely across regions. North America leads with salaries averaging $85,000–$105,000, while Europe offers £45,000–£60,000 in the UK, €55,000–€70,000 in Germany, and CHF 90,000–110,000 in Switzerland. Asia-Pacific salaries remain lower in absolute terms—India $20,000–$35,000, China $35,000–$50,000, and Singapore $55,000–$75,000—but are experiencing 10–15% annual growth. In the Middle East, salaries of $50,000–$70,000, often tax-free, make the region competitive. Regional differences reflect cost of living, research infrastructure, and regulatory environments. However, globalization and outsourcing are steadily narrowing the gap, especially for certified professionals with international expertise.
-
Pharmacovigilance Specialists generally earn less than CRAs or Clinical Trial Managers but more than entry-level coordinators. In 2025, PV specialists in the U.S. average $85,000–$105,000, while CRAs earn $95,000–$115,000 and Trial Managers $120,000–$145,000. Data Managers typically earn $95,000–$115,000, similar to CRAs. However, PV salaries grow faster in senior roles: Managers and Directors of Pharmacovigilance can earn $140,000–$180,000+, closing the gap with higher-tier roles. Specialists with certifications and experience in oncology or gene therapies often surpass the pay of mid-level CRAs, reflecting the increasing complexity of drug safety oversight.
-
The biggest factors influencing PV salaries are education, certification, experience, employer type, therapeutic area, and geography. Entry-level salaries are higher for those with master’s degrees or certifications like APRAC. Experience drives sharp increases: senior PV specialists in the U.S. exceed $120,000 annually, compared to entry-level pay of $65,000. Employer type also matters—pharma companies and global CROs pay significantly more than local organizations. Specialists in oncology, biologics, or rare diseases earn 15–25% premiums due to trial complexity. Finally, geography plays a large role: U.S. and Swiss salaries remain the highest, while Asia-Pacific and the Middle East show the fastest year-over-year growth.
Summing Up: Pharmacovigilance Opportunities
The pharmacovigilance salary landscape in 2025 reflects the growing importance of drug safety and regulatory compliance worldwide. Salaries are climbing steadily across all regions, with North America and Switzerland offering the highest pay, while Asia-Pacific and the Middle East show the fastest growth. Specialists skilled in biologics, oncology, and advanced therapies are commanding 15–25% higher compensation, proving the financial advantage of specialization.
Career growth in pharmacovigilance is equally promising. Entry-level roles provide competitive salaries and exposure to global regulatory systems, while mid-level and senior professionals advance into leadership positions exceeding $150,000–$180,000 annually in mature markets. Diversification into regulatory affairs, safety data science, and consultancy further expands opportunities for experienced specialists.
For graduates and experienced professionals alike, 2025 represents one of the strongest moments to enter or advance in pharmacovigilance. With rising demand, expanding pipelines, and global salary growth, this field offers not just stability but also exceptional long-term career rewards.