Clinical Research Associate (CRA) Salaries Worldwide 2025 Data Report
The Clinical Research Associate (CRA) role remains one of the most in-demand positions in clinical research, balancing site oversight, compliance, and data integrity across global trials. In 2025, CRA salaries are climbing as sponsors and CROs face shortages of qualified professionals capable of managing decentralized, multi-country studies. Employers are offering higher compensation and enhanced benefits to attract and retain CRAs, especially in oncology, rare disease, and adaptive trial designs where oversight is more complex.
This salary report provides a global perspective on CRA compensation, examining pay scales across North America, Europe, and Asia-Pacific. It highlights the variations in salary by region, the impact of employer type, and the influence of trial complexity on earning potential. With data showing consistent upward salary trends, CRAs are among the top earners outside physician-led positions in clinical research.
CRA Role and Industry Importance
Core Responsibilities of CRAs
The Clinical Research Associate (CRA) ensures clinical trials are conducted according to protocol, Good Clinical Practice (GCP), and regulatory standards. CRAs monitor data accuracy, verify patient safety measures, and conduct site visits to ensure compliance. In 2025, CRAs are also managing decentralized and hybrid trials, which require oversight of remote monitoring systems and electronic data capture (EDC). Their role ensures sponsors and regulators can trust trial data for drug approvals.
Importance in Trial Success
CRAs are central to data integrity and patient safety, making their oversight one of the most critical aspects of clinical research. Without CRAs, trial sponsors face risks of data rejection, regulatory penalties, or patient safety lapses. They act as quality controllers, ensuring that investigators and site teams adhere to approved processes. Their independence and ability to audit trial conduct in real time make them indispensable for study validation and credibility.
Industry Demand in 2025
Global demand for CRAs has surged, with shortages particularly acute in oncology, immunology, and rare disease trials. As clinical studies expand across continents, CRAs are tasked with managing multiple sites simultaneously. Salaries are rising in response, with average pay growth of 10–15% since 2023 across most regions. CROs, in particular, are aggressively hiring, often offering bonuses or flexible work arrangements to retain talent. This demand underscores the CRA’s strategic importance to the industry’s growth.
Career Impact and Pathway
The CRA role is often considered a gateway to higher-paying positions such as Senior CRA, Clinical Trial Manager, or Project Manager. The experience gained in regulatory compliance, patient safety, and multi-site coordination provides a foundation for career advancement. With CRA demand increasing worldwide, professionals entering the role in 2025 are positioned for strong salary growth and long-term stability. Employers continue to view the CRA role as mission-critical, ensuring competitive compensation packages and career mobility for qualified candidates.
Salary Ranges by Region
North America
In North America, CRA salaries remain among the highest globally in 2025. In the United States, the average CRA earns $95,000–$115,000 annually, with Senior CRAs often surpassing $130,000. Top-paying cities include Boston, San Francisco, and New York, driven by biotech concentration and higher living costs. In Canada, salaries are slightly lower, averaging CAD 80,000–100,000, though benefits packages tend to be stronger. Clinical Research Associates in oncology and rare disease trials earn 15–20% more than peers due to trial complexity. CROs are competing aggressively with pharma companies, offering retention bonuses and flexible work options to reduce turnover. North America continues to set the benchmark for CRA salaries worldwide.
Remote monitoring has further expanded opportunities, allowing CRAs outside major hubs to secure higher salaries than in previous years. Certified CRAs, particularly those holding the CCRPS CRA Certification, often earn 10–15% above the median, reflecting employer demand for credentialed professionals. As decentralized trial adoption accelerates, demand for CRAs with digital platform experience is surging. This trend ensures consistent salary growth, even in regions where pay levels have already plateaued at high baselines. For professionals seeking top-tier compensation, the U.S. and Canada remain the most lucrative CRA markets.
Europe
European CRA salaries vary widely depending on the country. In the United Kingdom, CRAs typically earn £45,000–£60,000, with Senior CRAs reaching £70,000+. In Germany, salaries range from €55,000–€75,000, reflecting its strong clinical research infrastructure. Switzerland remains the leader, where CRAs earn CHF 100,000–120,000, setting the highest European benchmark. France, Spain, and Italy fall slightly below Germany, averaging €45,000–€65,000 annually. Multilingual CRAs are in high demand across the continent, with bilingual or trilingual professionals often earning 10–20% more due to cross-border trial management responsibilities.
Regulatory complexity across the European Union also influences compensation. CRAs with advanced knowledge of EMA regulations or experience managing multinational trials are positioned for faster promotions and higher salaries. Brexit has created added competition in the UK, with employers offering retention bonuses to avoid talent loss. Switzerland continues to attract cross-border CRAs from Germany and France, thanks to significantly higher wages. Overall, Europe provides strong earning potential, though salary differences between countries remain wider than in North America.
Asia-Pacific
The Asia-Pacific region is experiencing the fastest CRA salary growth in 2025. In India, salaries average $30,000–$45,000 annually, but year-over-year growth exceeds 15%, making it one of the most rapidly advancing markets. In China, CRAs earn $40,000–$60,000, with top-tier professionals at multinational CROs exceeding $70,000. Singapore leads the region, offering $70,000–$90,000, aligning closely with mid-tier Western benchmarks. Australia is another strong market, with CRAs earning AUD 80,000–110,000, reflecting its mature trial infrastructure.
Rapid expansion of clinical trials in oncology, vaccines, and biologics is fueling CRA demand across Asia-Pacific. Salaries are rising fastest for CRAs experienced in decentralized trials and digital compliance platforms. Employers are also offering relocation packages and bonuses to attract international talent, particularly in Singapore and China. Certification is a critical differentiator: CRAs holding the CCRPS CRA Certification are able to command higher salaries, often closing the gap with U.S. peers. Asia-Pacific may not yet match North America in absolute pay, but its double-digit growth trajectory makes it one of the most promising regions for CRA career advancement and compensation.
| Region | Average CRA Salary (2025) | Senior CRA Salary | Notes |
|---|---|---|---|
| North America | $95,000 – $115,000 (U.S.) CAD 80,000 – 100,000 (Canada) |
$130,000+ | Highest global benchmark; oncology trials pay 15–20% more |
| Europe | £45,000 – £60,000 (UK) €55,000 – €75,000 (Germany) CHF 100,000 – 120,000 (Switzerland) |
£70,000+ / €80,000+ / CHF 130,000+ | Switzerland leads; multilingual CRAs earn premiums |
| Asia-Pacific | $30,000 – $45,000 (India) $40,000 – $60,000 (China) $70,000 – $90,000 (Singapore) AUD 80,000 – 110,000 (Australia) |
$70,000+ / AUD 120,000+ | Fastest YOY growth (10–15%); CRO expansion drives demand |
Key Factors Affecting CRA Salaries
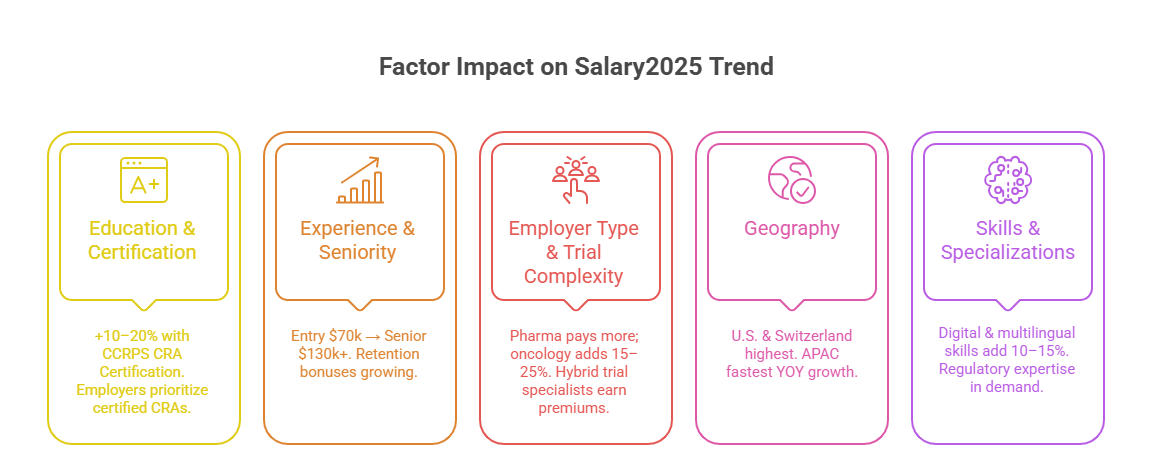
Education & Certification
Educational background and certification remain primary drivers of CRA salaries. While a bachelor’s degree in life sciences is standard, advanced degrees or industry-recognized credentials raise earning potential. The CCRPS CRA Certification is one of the strongest salary boosters in 2025, providing 10–20% higher pay compared to non-certified CRAs. Employers value certified professionals because they demonstrate regulatory literacy, GCP knowledge, and readiness to manage decentralized trials. In competitive markets like North America and Europe, certification is often the differentiator in promotions to Senior CRA or Trial Manager positions, accelerating both salary and career progression.
Experience & Seniority
Experience is a significant factor in determining CRA salaries. Entry-level CRAs in the U.S. earn around $70,000–$85,000, while mid-level CRAs with 3–5 years of monitoring experience rise to $95,000–$115,000. Senior CRAs overseeing complex multi-country trials often exceed $130,000. Employers pay premiums for professionals with a proven record of high-quality data monitoring, regulatory compliance, and site relationship management. With turnover remaining high, CROs are willing to pay more to retain experienced CRAs. Seniority also brings additional perks such as performance bonuses, flexible work arrangements, and relocation packages, further increasing total compensation.
Employer Type & Trial Complexity
Salaries vary widely depending on employer type and the complexity of trials managed. Pharmaceutical companies typically pay more than CROs, though CROs often provide faster career growth and performance incentives. CRAs working on oncology, rare disease, or gene therapy studies earn 15–25% higher pay than peers in general therapeutic areas. Trial complexity also influences workload and compensation: decentralized and hybrid studies require digital monitoring expertise, which commands higher salaries. Employers reward CRAs capable of adapting to advanced trial technologies, viewing them as essential assets for operational success.
Geography & Regional Market Demand
Geography plays a decisive role in CRA salaries. North America and Switzerland remain the highest-paying regions, but Asia-Pacific is experiencing the fastest year-over-year growth, with salaries increasing 10–15% annually. Countries like Singapore, China, and Australia are approaching Western benchmarks, particularly for certified CRAs. The Middle East also offers competitive packages with tax-free salaries and housing benefits. Global mobility is an increasing trend, with experienced CRAs securing higher pay by relocating to emerging markets where demand is outpacing supply. Location continues to determine baseline salaries, but globalization is gradually narrowing the gaps.
Skills & Specializations
Specialized skills significantly affect salary levels. CRAs proficient in electronic data capture (EDC), risk-based monitoring, and regulatory reporting consistently earn higher salaries. Bilingual and trilingual CRAs are especially valuable in Europe, Asia-Pacific, and Africa, where cross-border trial management requires multilingual communication. In 2025, digital proficiency is increasingly rewarded—CRAs experienced with decentralized trial platforms earn 10–15% above peers. Skills in oncology or immunology trials also push salaries higher due to the complexity of therapeutic areas. The trend is clear: specialization and adaptability are key to maximizing CRA earning potential worldwide.
Career Progression: CRA to Senior CRA & Manager
From CRA to Senior CRA
The first major career milestone for a Clinical Research Associate (CRA) is progression to Senior CRA, typically after 3–5 years of monitoring experience. Salaries rise sharply, averaging $115,000–$135,000 in the U.S., compared to $95,000–$115,000 for mid-level CRAs. Senior CRAs oversee more complex trials, manage junior CRAs, and often handle global sites. Employers prioritize those with strong regulatory knowledge, proven site relationship management, and advanced certifications. The CCRPS CRA Certification accelerates this transition, reducing the time to promotion and boosting earning potential by 10–20%.
Transition to CRA Manager or Trial Manager
After advancing to Senior CRA, the next step is often CRA Manager or Clinical Trial Manager, where salaries exceed $140,000–$160,000 annually. These roles involve leadership responsibilities such as team supervision, budget management, and cross-country trial oversight. Trial Managers act as the strategic link between sponsors and site teams, making leadership, communication, and regulatory expertise critical. Professionals who demonstrate project management skills, especially those with PMP credentials, can move into these roles faster. Career progression from CRA to management unlocks both higher pay and long-term stability.
Long-Term Leadership Pathways
For CRAs aiming at executive-level roles, career progression doesn’t stop at trial management. Many Senior CRAs transition into Director of Clinical Operations or Project Director positions, earning $180,000–$200,000+ depending on the region. These roles demand a blend of operational leadership, regulatory strategy, and global trial coordination. Some professionals also move into medical affairs or regulatory affairs leadership, leveraging CRA experience in compliance and data integrity. In 2025, employers see CRAs as a primary pipeline for operational leadership, making the career pathway both lucrative and stable.
The Role of Certification in Career Progression
Certification remains a critical accelerator at every stage of CRA career development. The CCRPS CRA Certification not only boosts salaries but also shortens the timeline to promotions, particularly in competitive regions like North America and Europe. Certified CRAs are more frequently chosen for leadership roles, as employers value validated competency. For professionals looking to advance quickly from entry-level CRA to management, certification provides a clear advantage—translating into higher pay, international mobility, and long-term career growth.
Global Trends Shaping CRA Pay
Rising Demand for Decentralized Trial Expertise
One of the strongest global trends influencing CRA pay in 2025 is the adoption of decentralized and hybrid trials. CRAs skilled in remote monitoring, digital platforms, and electronic data capture (EDC) systems are in short supply. Employers are offering premiums of 10–15% above median salaries to professionals with digital trial expertise. As sponsors shift toward patient-centric study models, CRAs who can manage both traditional site visits and virtual oversight will continue to command higher pay worldwide.
Regional Salary Convergence
Another key trend is regional salary convergence. While North America and Switzerland remain the top-paying markets, Asia-Pacific is experiencing double-digit annual growth in CRA salaries. Countries like Singapore and China are approaching Western pay levels, narrowing the gap. Middle Eastern countries, particularly the UAE and Saudi Arabia, are also offering competitive packages with tax-free benefits. This trend signals that global CROs are standardizing compensation across regions, making relocation and cross-border assignments more attractive for CRAs.
Employer Competition for Talent
Competition among CROs, pharma companies, and biotech firms is another global driver of CRA salaries. CROs are aggressively matching or exceeding pharma salaries to retain staff, as turnover remains one of the costliest challenges in clinical research. Employers are supplementing base pay with retention bonuses, performance incentives, and flexible work arrangements. This competitive environment ensures steady salary growth for CRAs, especially in regions with workforce shortages.
Specialization and Therapeutic Areas
Specialization is shaping CRA pay globally. CRAs working in oncology, rare diseases, or advanced therapies such as cell and gene treatments earn 15–25% higher salaries than peers in general therapeutic areas. Regulatory agencies demand tighter compliance for these trials, making experienced CRAs indispensable. As complex therapies dominate the clinical pipeline, specialized CRAs will see the strongest compensation growth. This specialization trend is global, impacting salaries across North America, Europe, and emerging markets alike.
| Global Trend | Impact on CRA Salaries | 2025–2030 Outlook |
|---|---|---|
| Decentralized & Hybrid Trials | +10–15% premium for CRAs with digital trial expertise | Growing demand for EDC & remote monitoring skills |
| Regional Salary Convergence | Asia-Pacific & Middle East salaries rising fast | Closing gap with North America & Switzerland |
| Employer Competition | CROs, pharma, and biotech firms offering bonuses & flexibility | Retention incentives drive salary growth |
| Specialization in Oncology & Rare Diseases | 15–25% higher pay for CRAs in high-complexity trials | Strongest compensation growth expected in oncology |
How CCRPS CRA Certification Boosts Salaries and Career Growth
Certification as a Salary Multiplier
The CCRPS CRA Certification has become a key differentiator in the competitive 2025 job market. Certified Clinical Research Associates consistently earn 10–20% higher salaries than non-certified peers, reflecting employer trust in validated GCP and regulatory knowledge. For entry-level CRAs, certification accelerates job placement and starting pay, while experienced CRAs leverage it for promotions to Senior CRA or Trial Manager roles. In regions like Asia-Pacific and the Middle East, certification often narrows the pay gap with North America, making it one of the strongest salary multipliers globally.
Career Advancement and Mobility
Beyond salary, CCRPS certification accelerates career mobility and international opportunities. Employers value certified CRAs when filling roles in global CROs and multinational pharma companies. Certification ensures readiness for managing complex cross-border trials, giving certified CRAs access to relocation packages, bonuses, and leadership opportunities. This advantage is especially pronounced in emerging regions, where certified professionals often secure pay scales closer to Western benchmarks. Certification thus expands both earning potential and global career reach.
Standing Out in a Competitive Market
The CRA job market in 2025 is competitive, with employers struggling to retain experienced staff. The CCRPS CRA Certification signals proven competency, reducing the need for intensive training and lowering compliance risks. Certified CRAs are frequently prioritized for high-demand therapeutic areas like oncology and rare diseases, which offer 15–25% higher pay. For professionals targeting leadership positions, certification shortens the promotion timeline and strengthens negotiation leverage for salaries and benefits.
Long-Term Career Impact
Certification is not just about short-term salary boosts—it creates a foundation for long-term career growth. Certified CRAs transition faster into Senior CRA, Trial Manager, or Clinical Operations Director roles, many of which exceed $150,000–$180,000 annually. As trials become more global and technology-driven, certified professionals are consistently seen as the most adaptable and reliable hires. In short, CCRPS certification is one of the most effective career investments for CRAs aiming to maximize both immediate pay and future leadership opportunities.
Frequently Asked Questions
-
The average salary for a Clinical Research Associate (CRA) in 2025 varies by region. In the U.S., CRAs earn $95,000–$115,000 annually, with Senior CRAs exceeding $130,000. In Canada, salaries range from CAD 80,000–100,000, often paired with stronger benefits. European salaries differ: the UK averages £45,000–£60,000, Germany €55,000–€75,000, and Switzerland leads with CHF 100,000–120,000. In Asia-Pacific, salaries are lower but growing fast: India averages $30,000–$45,000, China $40,000–$60,000, and Singapore $70,000–$90,000. Globally, CRAs are experiencing consistent salary growth, fueled by trial expansion and rising demand for compliance expertise.
-
In 2025, the highest-paying countries for CRAs are the United States, Switzerland, and Singapore. U.S. CRAs average $95,000–$115,000, with top earners surpassing $130,000. Switzerland offers the strongest European pay at CHF 100,000–120,000, attracting CRAs from neighboring countries. Singapore leads Asia-Pacific with salaries of $70,000–$90,000, reflecting its role as a clinical research hub. Germany, Canada, and Australia also pay competitively, though slightly below these leaders. By contrast, India and Brazil pay lower base salaries but are showing 10–15% year-over-year growth, narrowing the gap with Western benchmarks. Certified CRAs, especially with the CCRPS CRA Certification, secure higher salaries in every region.
-
The salary gap between entry-level and Senior CRAs is significant in 2025. In the U.S., entry-level CRAs earn around $70,000–$85,000, while Senior CRAs average $115,000–$135,000, sometimes exceeding $140,000 in oncology or global trials. In Europe, entry-level CRAs earn €40,000–€55,000, while Senior CRAs can exceed €75,000. Asia-Pacific shows similar jumps, with India’s Senior CRAs earning $50,000+, nearly double entry-level pay. Employers pay premiums for senior staff due to their expertise in risk-based monitoring, data integrity, and site relationship management. Certification and experience in high-demand therapeutic areas accelerate progression to Senior CRA roles, unlocking higher salaries and benefits packages.
-
The key factors influencing CRA salaries in 2025 include education, certification, experience, geography, employer type, and therapeutic specialization. Certified CRAs, particularly with the CCRPS CRA Certification, earn 10–20% more than peers. Experience is critical: salaries rise sharply after 3–5 years in the role, especially with multi-country trial oversight. Employer type also matters—pharma companies typically pay more than CROs, though CROs offer faster promotions. Specialization in oncology, immunology, or rare diseases boosts pay by 15–25%, reflecting the complexity of these trials. Geography is another driver, with North America and Switzerland paying the most, while Asia-Pacific and the Middle East show the fastest growth.
-
CRA salaries differ widely across regions. In North America, salaries average $95,000–$115,000 in the U.S. and CAD 80,000–100,000 in Canada. Europe shows strong variation: the UK averages £45,000–£60,000, Germany €55,000–€75,000, and Switzerland CHF 100,000–120,000. Asia-Pacific offers lower base salaries but faster growth: India averages $30,000–$45,000, China $40,000–$60,000, and Singapore $70,000–$90,000. Middle Eastern countries like the UAE offer $65,000–$90,000 tax-free, often with housing allowances. Regional differences reflect trial density, regulatory frameworks, and cost of living. Despite disparities, globalization is narrowing gaps, with Asia-Pacific and Middle East salaries rising rapidly toward Western levels.
-
Certification significantly enhances CRA salary potential. Employers prefer certified CRAs because they reduce training costs and ensure compliance with GCP and regulatory standards. The CCRPS CRA Certification is widely recognized, boosting salaries by 10–20% globally. Certified CRAs are often prioritized for complex trials, multinational assignments, and promotions to Senior CRA or Trial Manager positions. In regions like India, China, and Brazil, certification is often the deciding factor in securing roles with multinational CROs, raising salaries closer to U.S. benchmarks. Beyond salary, certification accelerates career progression, allowing CRAs to move into leadership roles faster and with stronger negotiation leverage.
Final Thoughts
The Clinical Research Associate salary landscape in 2025 highlights a role that has become indispensable to global clinical trials. CRAs ensure compliance, patient safety, and data integrity across increasingly complex, decentralized, and multi-country studies. Salaries reflect this importance, with North America and Switzerland setting the highest benchmarks, while Asia-Pacific and the Middle East show the fastest growth trajectories.
Career progression is equally rewarding. Entry-level CRAs quickly advance into Senior CRA or Trial Manager positions, often doubling their salaries within a few years. Specialization in oncology or rare diseases, combined with digital trial expertise, positions CRAs for the strongest compensation growth. Employers are competing aggressively to retain talent, offering not only higher pay but also benefits like retention bonuses and flexible work arrangements.