Phase III Clinical Trials: Definitive Guide & Case Studies
Clinical trials form the backbone of modern drug development, but Phase III trials are the defining battleground where a product either advances to approval or fails the ultimate test. At this stage, investigational therapies are tested in large-scale populations, typically ranging from hundreds to thousands of participants, across multiple geographies. The stakes are immense—both clinically and financially. Drugs entering Phase III have a 50–60% chance of approval, but this phase consumes the largest share of R&D budgets and can span several years. It’s where the precision of data, rigor of design, and clarity of endpoints come under regulatory microscopes.
This guide delivers more than just an overview. We break down critical components of Phase III trials—from trial design, endpoint structuring, and global compliance, to patient engagement and data monitoring. You’ll explore real-world case studies like the Pfizer-BioNTech COVID-19 vaccine trial and Biogen’s controversial Alzheimer’s drug, Aducanumab, to understand what success and failure look like in practice. Whether you're a sponsor, investigator, or aspiring clinical research professional, this guide offers granular insights tied directly to operational execution. Each section has been crafted with high-intent keywords to ensure relevance and discoverability for those ready to lead in this space.
The Role of Phase III in the Clinical Trial Lifecycle
Phase III trials are the final and most critical evaluative stage before a drug or biologic reaches the market. In the broader context of Phases I through IV, Phase I establishes safety and pharmacokinetics in healthy volunteers, while Phase II tests preliminary efficacy and side effects in small patient groups. Phase III, however, determines if a treatment is viable at scale, not just under idealized conditions but in broader, real-world populations.
At this point, investigational products must demonstrate statistically significant, clinically meaningful outcomes. Trial sizes often exceed 1,000 participants, with designs built for geographic, racial, and age diversity to reflect the future prescribing population. These trials can involve 100+ sites across multiple countries, which escalates costs and logistical complexity.
Phase III is also the most expensive phase, absorbing over $100 million on average per trial, with high-cost outliers reaching up to $500 million. These trials consume over 60% of total drug development budgets and can span 3 to 7 years, depending on enrollment rates, regulatory feedback, and endpoint durations. Delays in this phase translate into lost revenue potential or complete market failure.
The outcomes of Phase III form the basis for New Drug Applications (NDAs), Biologics License Applications (BLAs), and Marketing Authorization Applications (MAAs). Regulators require compelling evidence not only of efficacy but also of safety across varied demographics. Failure to meet primary endpoints—despite earlier-phase promise—often leads to program discontinuation, making this stage the highest-stakes investment in the lifecycle.
Additionally, Phase III trials bridge the gap between controlled environments and real-world application. Patients in this phase may be on other medications, have comorbidities, or vary in adherence—factors that mirror actual clinical practice. For sponsors and CROs, the focus shifts from proof-of-concept to scalability, compliance, and market readiness.
Without a successful Phase III trial, no drug can reach commercial approval. It is the ultimate test of a product’s potential—and the defining measure of clinical development success.
Core Design Elements of Phase III Trials
Randomization & Blinding Structures
Randomization and blinding are critical to Phase III trial integrity, minimizing biases that could distort efficacy and safety outcomes. Randomization assigns participants to treatment or control groups using robust computer-generated algorithms, ensuring groups are comparable and selection bias is minimized. Often, stratified randomization balances key prognostic factors—such as age, gender, disease severity, or geographic region—across arms to reduce variability and improve statistical power.
Blinding maintains objectivity during treatment administration and outcome assessment. In double-blind trials, neither the investigator nor the participant knows the assigned treatment, effectively reducing placebo effects and observer bias. Single-blind trials keep patients unaware but allow investigators to know the assignment, which may introduce subtle biases but are used when double-blinding is operationally infeasible. Ensuring blinding integrity through monitoring and audits is essential to preserve data credibility.
Double-Blind vs. Single-Blind
Double-blind designs remain the gold standard for Phase III trials, especially when subjective endpoints such as pain relief, mood, or quality of life are measured. They minimize bias on both the investigator and participant sides, increasing reliability. Single-blind designs are chosen when double-blinding is impractical due to factors like different administration routes or distinctive side effects. Regulatory agencies require strong justification for single-blind use and often impose additional safeguards to limit investigator bias, including independent endpoint adjudication.
Control Arms & Placebos
Control groups serve as benchmarks for evaluating efficacy and safety. Phase III trials use either placebo controls or active comparators, selected based on ethical considerations and disease context. For severe or life-threatening conditions, active-controlled trials are mandatory to ensure no patient is deprived of effective therapy. Placebos are reserved for scenarios lacking approved treatments and where withholding therapy does not pose excessive risk. Placebo formulations are meticulously matched in appearance, taste, and administration route to maintain blinding. Trials may also adopt unequal randomization ratios (e.g., 2:1) favoring the investigational arm to enhance recruitment without compromising statistical validity.
Patient Selection & Sample Sizing
Patient selection directly impacts the trial’s external validity and chances of regulatory approval. Trials with inappropriate or overly narrow criteria risk underpowered outcomes or poor generalizability, while excessively broad criteria may introduce confounders.
Inclusion/Exclusion Criteria
Inclusion criteria specify participant characteristics needed for eligibility, such as confirmed diagnosis, relevant biomarkers, or disease stage, ensuring a homogenous, scientifically meaningful population. Exclusion criteria filter out patients with confounders like comorbidities, interacting medications, or abnormal lab results to reduce variability and safety risks. While strict criteria increase internal validity, they may limit applicability to the broader patient population, a key concern for regulators. Agencies emphasize diverse representation, especially in diseases with known demographic variability. Ethical recruitment mandates transparent, fair criteria that comply with Good Clinical Practice (GCP) and avoid unjust exclusion.
Power Calculations & Sample Justification
Sample size determination is based on power calculations estimating the number of participants required to detect a clinically meaningful effect with high confidence. Typical power targets range from 80% to 90%, limiting false negatives. Calculations incorporate expected dropout rates, event incidence, and outcome variability. Regulators demand clear, data-driven assumptions, often relying on Phase II data or epidemiological studies. Overestimating effect sizes risks underpowered trials vulnerable to failure, whereas underestimating inflates costs and exposes more patients than necessary. Sponsors must strike a balance between statistical rigor and practical feasibility, considering recruitment capacity and budget constraints.
Endpoints & Outcome Definitions
Endpoints define success criteria and drive regulatory approval decisions. They must be clinically relevant, measurable, and pre-specified in the protocol to avoid bias.
Primary vs. Secondary Endpoints
Primary endpoints are the main efficacy measures, such as overall survival, symptom reduction, or disease progression-free intervals. These require rigorous statistical validation and prior regulatory approval to ensure they reflect meaningful clinical benefit. Secondary endpoints capture additional effects, including quality of life, biomarker responses, or functional improvements, often supporting broader claims. Trials use hierarchical testing strategies to control type I error when multiple endpoints are assessed. Failure to meet the primary endpoint can invalidate the trial, making endpoint selection critical. Alignment with FDA and EMA guidance during design is essential to avoid costly protocol amendments or regulatory setbacks.
Patient-Centric Outcome Tracking
Regulators and payers increasingly demand evidence on real-world impact. Patient-reported outcomes (PROs) measure subjective experiences like pain, fatigue, daily functioning, and quality of life, complementing clinical endpoints. Validated instruments such as EQ-5D, SF-36, or disease-specific scales standardize assessments, enabling cross-trial comparability. Integrating PROs strengthens reimbursement and market access strategies by demonstrating tangible patient benefit. However, PRO data require rigorous methodology, including cultural adaptation, consistent administration, and data quality monitoring. Comprehensive site training ensures accurate, reliable PRO collection, supporting regulatory acceptance and payer confidence.
| Core Design Element | Description | Key Considerations |
|---|---|---|
| Randomization & Blinding | Assigns participants to groups using algorithms; ensures objectivity by masking treatment assignments. | Double-blind for maximal bias reduction; single-blind only when double-blind isn’t feasible. |
| Control Arms & Placebos | Benchmarks efficacy and safety using placebo or active comparators. | Active controls for severe conditions; placebos for scenarios without approved therapies. |
| Patient Selection | Defines inclusion and exclusion criteria to target appropriate patient populations. | Balance between internal validity and real-world applicability; ensure diversity and fairness. |
| Sample Sizing | Determines participant numbers to ensure sufficient statistical power. | Based on effect size estimates, dropout rates, and variability; must align with regulatory expectations. |
| Endpoints | Specifies primary and secondary outcome measures to assess treatment success. | Pre-specified, clinically relevant, statistically validated; align with regulatory guidance. |
| Patient-Centric Outcomes | Uses PROs to capture patient experiences like pain, fatigue, and quality of life. | Requires validated instruments, standardized administration, and site training for data integrity. |
Regulatory Oversight & Global Compliance Pathways
US FDA: 21 CFR Parts 312 & 314
The U.S. Food and Drug Administration (FDA) regulates Phase III clinical trials under 21 CFR Parts 312 and 314, which govern Investigational New Drug (IND) applications and New Drug Applications (NDA). Sponsors must submit detailed preclinical data, chemistry and manufacturing controls, and a clinical protocol outlining study design, endpoints, eligibility criteria, and safety monitoring. This rigorous submission ensures scientific validity and patient safety before trials begin.
Throughout Phase III, the FDA enforces strict oversight by reviewing ongoing safety reports, adverse event documentation, and adherence to the approved protocol and Good Clinical Practice (GCP) standards. Sponsors typically engage in pre-IND and end-of-Phase II meetings to align trial design with FDA expectations, reducing the risk of delays or non-approval. Accelerated approval pathways and breakthrough therapy designations allow earlier approval based on surrogate or intermediate endpoints, with mandatory confirmatory Phase IV studies to verify long-term benefits and safety.
Interim analyses and independent Data Safety Monitoring Boards (DSMBs) play pivotal roles in large Phase III trials, enabling early detection of safety issues or compelling efficacy data. These mechanisms allow ethical trial modifications, early stopping for benefit or harm, or enhanced data collection as necessary. Failure to maintain compliance with FDA regulations or data integrity can result in clinical holds, delayed approvals, or rejection of marketing applications.
EMA & Global Harmonization (ICH-GCP)
The European Medicines Agency (EMA) oversees Phase III clinical trials within the European Union and enforces compliance with International Council for Harmonisation Good Clinical Practice (ICH-GCP) guidelines. ICH-GCP establishes a unified global standard for trial design, ethical conduct, informed consent, and data integrity, reducing duplication of regulatory efforts and facilitating multinational trial conduct.
Trials conducted in the EU must be registered in the EudraCT database, ensuring transparency and public accountability. Approval from national competent authorities and ethics committees in participating member states is mandatory before trial commencement. The EMA mandates comprehensive safety monitoring, including ongoing pharmacovigilance and detailed risk management plans, ensuring continuous assessment of patient safety throughout the trial lifecycle.
The EMA framework supports streamlined multinational trials and accelerated review pathways. Conditional marketing authorizations, similar to FDA accelerated approvals, enable earlier patient access to promising therapies while requiring sponsors to conduct post-approval safety studies. This balance promotes public health benefits alongside rigorous safety oversight.
Local Regulatory Adjustments (India, China, Brazil)
Conducting Phase III trials globally requires adapting to local regulatory frameworks in emerging markets such as India, China, and Brazil, which provide access to large, diverse patient populations but present unique regulatory challenges.
In India, the Central Drugs Standard Control Organization (CDSCO) regulates clinical trials. CDSCO mandates detailed trial applications including protocols, investigator brochures, ethics approvals, and local language informed consent forms. India enforces strict compensation policies for trial-related injuries and has recently streamlined timelines; however, regulatory requirements continue to evolve, demanding continuous vigilance from sponsors.
China’s National Medical Products Administration (NMPA) requires localized clinical data, often including Chinese patient cohorts, to demonstrate regional safety and efficacy. The NMPA performs site inspections to verify Good Clinical Practice adherence. Expedited pathways like priority review and breakthrough therapy designations facilitate faster access to innovative treatments. Early, sustained engagement with NMPA officials and local experts is critical for trial success.
In Brazil, the Agência Nacional de Vigilância Sanitária (ANVISA) integrates regulatory and ethical oversight with the National Commission for Ethics in Research (CONEP). Approval processes require submission of comprehensive documentation in Portuguese, local site participation, and compliance with data protection and patient rights laws. Brazil emphasizes the importance of local data generation and patient safety.
Effectively managing these local regulatory demands requires tailored strategies leveraging regional expertise, harmonized documentation, and flexible project management. Balancing global regulatory standards with local nuances ensures compliance without sacrificing efficiency, enabling timely regulatory approvals and broader patient access worldwide.
Poll: What’s the Biggest Challenge in Global Phase III Compliance?
Stakeholders Driving Phase III Execution
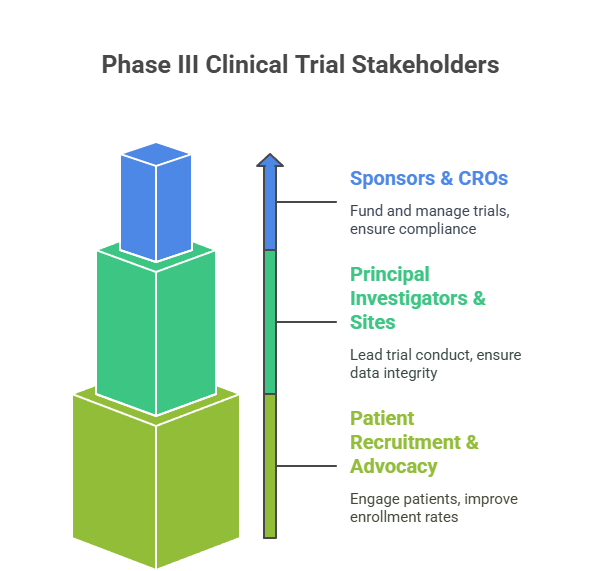
Successful Phase III clinical trials hinge on coordinated efforts among diverse stakeholders, each playing a distinct role in trial planning, execution, and oversight. Understanding these players and their responsibilities ensures streamlined operations and maximizes trial success.
Sponsors & CROs
Sponsors, typically pharmaceutical companies or biotechnology firms, fund and oversee clinical trials, setting scientific objectives, budgets, and regulatory compliance standards. Large pharmaceutical companies often maintain extensive internal clinical development teams with well-established processes and cross-functional coordination. In contrast, biotech startups frequently depend heavily on external partners due to limited internal resources.
Contract Research Organizations (CROs) serve as specialized execution partners, managing trial logistics, site coordination, patient recruitment, data collection, and regulatory submissions. CROs bring critical operational expertise, infrastructure, and scalability that enable sponsors to conduct complex, multi-region Phase III trials efficiently and in compliance with local regulations. Selecting a CRO with strong therapeutic area experience, proven track record, and global reach is vital for timely study completion, data quality, and regulatory success.
Principal Investigators & Sites
Principal Investigators (PIs) lead trial conduct at clinical sites, ensuring strict adherence to protocols, patient safety, and high-quality data integrity. Site selection is a strategic process, based on investigator expertise, access to target patient populations, clinical infrastructure, and historical performance metrics. Geographic diversity of sites enhances generalizability of results and regulatory acceptance but introduces logistical complexities that demand robust communication, monitoring, and quality assurance systems.
Patient Recruitment & Advocacy Support
Effective patient recruitment remains one of the most significant challenges in Phase III trials. Engagement models leverage targeted education tools, community outreach programs, and partnerships with patient advocacy organizations to improve enrollment rates and retention. Transparent and culturally sensitive informed consent processes, which respect patient autonomy and comply with ethical standards, build trust and facilitate compliance. Increasingly, sponsors employ digital recruitment platforms, social media campaigns, and real-world data analytics to identify and engage suitable candidates efficiently, reducing enrollment delays and supporting trial timelines.
Data Collection, Monitoring & Interim Analysis
Phase III trials generate vast volumes of data that demand rigorous collection methods, continuous monitoring, and strategic interim analyses to ensure data integrity, participant safety, and regulatory compliance.
Electronic Data Capture (EDC) Systems
Electronic Data Capture (EDC) platforms have become the industry standard for Phase III data collection. These systems enable real-time electronic data entry from clinical sites, centralized data storage, and automatic validation checks that drastically reduce manual errors and improve overall data accuracy. Leading EDC platforms provide comprehensive audit trails, which are essential for regulatory compliance, ensuring that every modification, entry, or correction is recorded and fully traceable. Integration capabilities with electronic health records (EHRs), laboratory information management systems (LIMS), and other clinical trial platforms improve data completeness, streamline workflows, and accelerate data cleaning processes. Modern EDCs support complex trial designs, including adaptive protocols, multi-arm studies, and decentralized clinical trials, enhancing operational flexibility.
Interim Analysis & Data Safety Monitoring Boards (DSMBs)
Interim analyses are pre-specified evaluations of accumulating trial data conducted at predetermined time points or enrollment milestones. These analyses assess trends in efficacy and safety signals to determine whether a trial should continue unchanged, be modified, or be stopped early due to overwhelming efficacy, futility, or safety concerns. Independent Data Safety Monitoring Boards (DSMBs), comprised of clinical experts and statisticians without direct trial involvement, oversee these interim analyses. DSMBs provide unbiased recommendations to sponsors and regulatory bodies, preserving patient safety and scientific integrity. Their role is critical in large, high-risk Phase III trials, where ethical considerations around exposing patients to potentially inferior or harmful treatments are paramount.
Adverse Event Tracking & Protocol Deviations
Robust adverse event (AE) tracking systems are essential to promptly identify, document, and report safety issues. These systems facilitate real-time escalation of serious adverse events (SAEs) and suspected unexpected serious adverse reactions (SUSARs) in compliance with FDA, EMA, and local regulatory timelines. Protocol deviations, which may impact data validity or patient safety, are also systematically tracked and managed through electronic systems and site monitoring visits. Proactive AE and deviation management help mitigate risks, maintain trial quality, and ensure regulatory requirements are met throughout the trial lifecycle.
Data Collection, Monitoring & Interim Analysis
- Data Collection:
- Utilizes Electronic Data Capture (EDC) systems for real-time, accurate data entry and centralized storage.
- EDCs feature automatic validation checks, audit trails, and integration with EHRs, LIMS, and other platforms.
- Supports adaptive protocols, multi-arm designs, and decentralized trials for enhanced flexibility.
- Interim Analysis:
- Conducted at pre-specified timepoints to evaluate efficacy, safety, and trial progression.
- Facilitated by independent Data Safety Monitoring Boards (DSMBs) for unbiased oversight.
- May lead to trial continuation, modification, or early termination based on findings.
- Monitoring & Safety Tracking:
- Real-time adverse event (AE) tracking systems enable prompt identification and escalation of SAEs and SUSARs.
- Protocol deviations are tracked and managed systematically through electronic systems and site monitoring.
- Ensures regulatory compliance and maintains trial integrity.
Budget, Timelines & Risk Management
Effective management of budget, timelines, and risks is critical for successful Phase III clinical trials due to their complexity, scale, and substantial cost intensity.
Cost Breakdown
Phase III trials represent the most expensive stage in drug development, frequently accounting for 50-70% of total clinical development costs. Major cost drivers include patient recruitment, site management, investigator fees, clinical monitoring, data management, and regulatory compliance activities. Per-patient costs vary widely depending on the therapeutic area, geographic regions involved, and trial complexity but commonly range between $20,000 and $60,000 or more. Additional expenditures include manufacturing and distribution of investigational medicinal products (IMPs), pharmacovigilance infrastructure, centralized laboratory testing, and quality assurance audits. Unanticipated budget overruns can cause significant delays in regulatory submissions and negatively impact overall project return on investment (ROI).
Timelines & Delays
Phase III trials typically last 2 to 5 years, influenced by multiple factors including enrollment pace, site initiation timelines, and data cleaning processes. Patient enrollment delays remain a major bottleneck, often caused by strict inclusion/exclusion criteria, limited eligible patient populations, or competition with other trials. Regulatory review timelines and site activation challenges also contribute to extended study duration. Moreover, delays in database lock, query resolution, and final statistical analysis can postpone submission and approval timelines, directly affecting the product’s time to market. To mitigate such risks, sponsors increasingly adopt adaptive trial designs, real-time data monitoring, and innovative patient engagement strategies.
Operational Risk Mitigation
Proactive contingency planning and dynamic forecasting are essential for managing operational risks effectively. This includes strategies like backup site identification, diversified patient recruitment approaches, and application of agile project management principles. Early detection of potential bottlenecks through key performance indicators (KPIs) and risk assessment tools enables sponsors to adjust resource allocation and trial activities in real time. Additionally, risk-based monitoring (RBM) prioritizes critical data points and high-risk sites, optimizing oversight efforts while maintaining compliance, data quality, and participant safety.
Case Studies from the Field
Examining real-world Phase III trials provides valuable insights into the complexities, successes, and challenges inherent in this critical clinical development stage.
Pfizer-BioNTech COVID-19 Vaccine Trial
The Pfizer-BioNTech COVID-19 Phase III trial represents one of the most remarkable achievements in modern clinical research. With over 40,000 participants enrolled across diverse geographic regions, this trial employed a randomized, double-blind, placebo-controlled design to rigorously assess vaccine efficacy and safety. The trial’s rapid execution was facilitated by innovative collaboration among sponsors, contract research organizations (CROs), regulatory agencies, and clinical sites worldwide. Advanced digital tools such as electronic data capture systems, remote monitoring, and centralized databases enabled real-time data collection, oversight, and analysis. Adaptive elements allowed modifications based on interim findings, improving efficiency without compromising scientific integrity. The trial demonstrated a 95% efficacy rate in preventing symptomatic COVID-19, with a favorable safety profile, leading to Emergency Use Authorization (EUA) within months—a record timeline for vaccine development. This success not only accelerated global vaccine deployment but also established new operational benchmarks for Phase III trials conducted under pandemic conditions, highlighting the power of agility, technology, and global coordination.
Aducanumab in Alzheimer’s: When Phase III Fails
The Aducanumab Phase III trials highlight the intrinsic challenges and uncertainties in developing therapies for complex diseases such as Alzheimer’s. Biogen initiated two large-scale, placebo-controlled Phase III trials designed to evaluate Aducanumab’s effect on slowing cognitive decline. Early futility analyses prompted trial termination due to a perceived lack of efficacy, triggering industry-wide scrutiny. However, a subsequent detailed reanalysis revealed statistically significant cognitive benefits in specific patient subgroups, particularly those with early-stage disease. This led to contentious debates around the validity of primary endpoints, the interpretation of subgroup data, and the rigor of interim analyses. Despite the controversy, the FDA granted conditional accelerated approval, emphasizing the unmet need for Alzheimer’s treatments and allowing patients early access pending further confirmatory studies. Aducanumab’s journey underscores the critical importance of robust trial design, precise endpoint selection, transparent data review, and regulatory dialogue. It also reflects the broader implications of Phase III outcomes on corporate strategy, patient advocacy, and the evolving landscape of neurodegenerative research.
| Case Study | Key Highlights | Lessons Learned |
|---|---|---|
| Pfizer-BioNTech COVID-19 Vaccine Trial |
- 40,000+ participants across regions - Randomized, double-blind, placebo-controlled - Rapid data collection with digital tools - Adaptive design with interim modifications - 95% efficacy, EUA granted |
- Collaboration & digital innovation accelerate timelines - Adaptive design enhances trial efficiency - Real-world agility and coordination critical in crises |
| Aducanumab in Alzheimer’s |
- Two large-scale placebo-controlled trials - Early termination, later subgroup benefits - Controversial endpoint validity - FDA conditional approval despite debate |
- Robust design and endpoint selection vital - Subgroup analysis can inform decisions - Regulatory dialogue and transparency are key |
How the Certified Clinical Research Professional (CCRP) Helps You Lead Phase III Trials
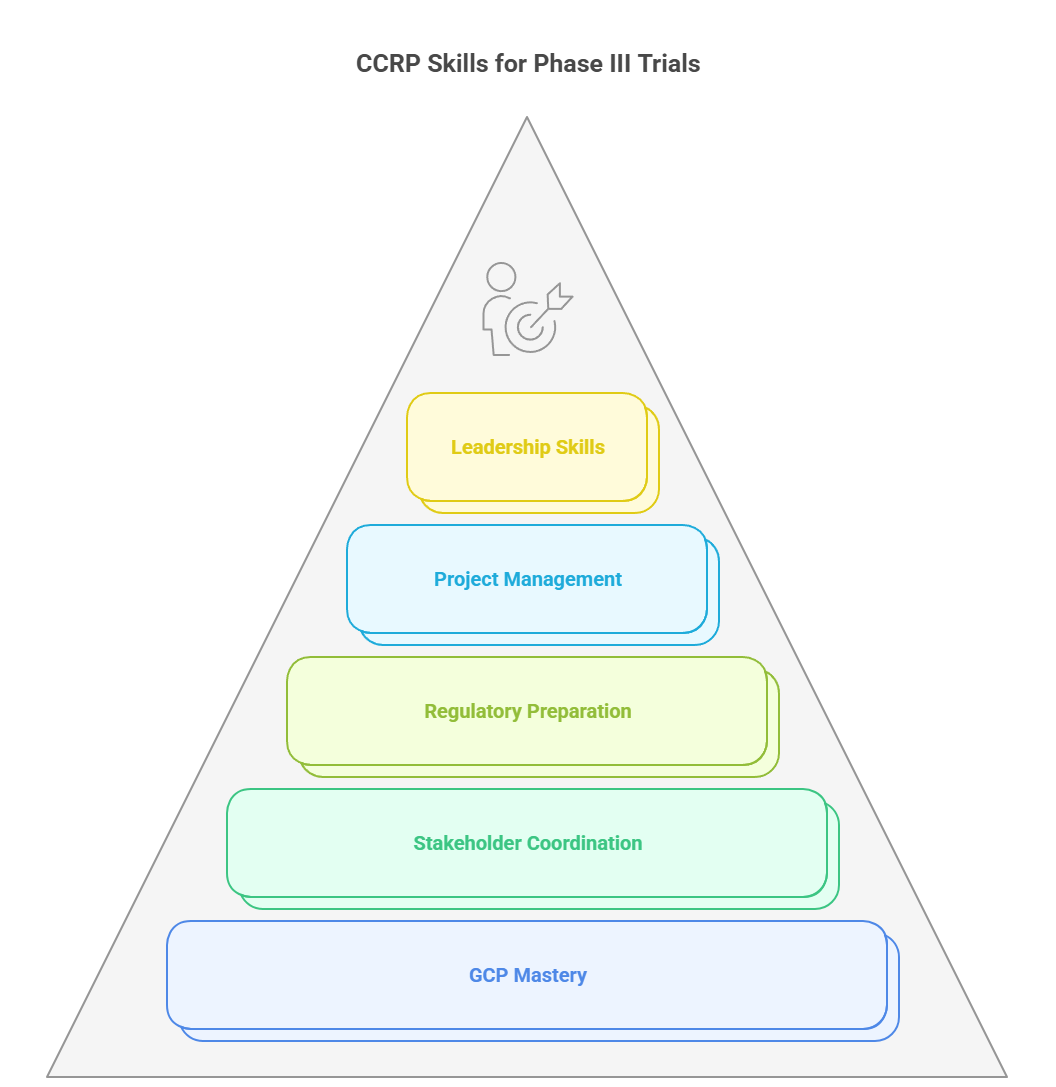
The Certified Clinical Research Professional (CCRP) credential provides targeted training to prepare clinical research professionals for the complex demands of Phase III trials. It focuses on the critical knowledge and skills needed to manage large, high-stakes studies efficiently, compliantly, and with strategic insight.
Course Modules Aligned to Real-World Trial Execution
The CCRP program covers essential areas such as trial design, patient recruitment, informed consent, and data management—all aligned with Good Clinical Practice (GCP) standards. It teaches practical strategies for managing diverse patient populations and complex trial protocols. The curriculum also includes modules on adaptive trial designs and interim analyses, helping professionals adapt to evolving data without compromising scientific rigor or regulatory expectations.
Emphasis on GCP, Stakeholder Management, Regulatory Preparation, and Project Management
Mastering GCP is fundamental in the CCRP course, ensuring trials meet international ethical and quality standards. Participants learn how to coordinate effectively with sponsors, CROs, investigators, and regulatory bodies to maintain smooth trial operations. Regulatory preparation training covers key submissions like IND applications and accelerated approval pathways. Additionally, the program strengthens project management skills, focusing on risk mitigation, budgeting, and timeline adherence to reduce delays and cost overruns.
By earning the Certified Clinical Research Professional credential, you gain not only technical expertise but also leadership skills to guide Phase III trials from planning through successful completion. This certification elevates your ability to deliver results, manage complex challenges, and accelerate drug development timelines.
Explore the Certified Clinical Research Professional course to get trained on Phase III execution and elevate your clinical research career with industry-recognized expertise.
Final Thoughts
Phase III clinical trials are the critical junction where promising therapies face their ultimate test before reaching patients. This stage demands rigorous design, meticulous execution, and seamless collaboration across multiple stakeholders. The scale, complexity, and resource intensity of Phase III trials make them the most challenging yet the most rewarding segment of drug development.
Investing in advanced training like the Certified Clinical Research Professional (CCRP) certification significantly enhances your ability to lead these trials with confidence and precision. Certified professionals possess a robust understanding of regulatory frameworks, ethical standards, and operational best practices—factors essential for navigating the intricacies of Phase III studies.
In today’s competitive clinical research landscape, being certified translates into measurable ROI by reducing trial delays, minimizing compliance risks, and improving data quality. Moreover, it positions you as a strategic leader capable of steering trials toward successful completion, accelerating the path to market approval, and ultimately benefiting patients worldwide.
Phase III trials may be complex and costly, but with the right expertise and certification, they become opportunities to drive innovation and make a meaningful impact on healthcare outcomes. Pursuing mastery in this domain is not just a career investment—it’s a commitment to advancing science and improving lives.
Frequently Asked Questions.
-
Phase III clinical trials are the definitive step that confirms a drug’s safety and efficacy across large, diverse patient populations. Unlike earlier phases that focus on safety (Phase I) and preliminary efficacy (Phase II), Phase III provides comprehensive data demonstrating the therapeutic benefit and risk profile necessary for regulatory approval. These trials also evaluate how the drug performs across different demographics and co-morbidities, ensuring real-world applicability. Without successful Phase III results, new treatments cannot be approved for widespread clinical use.
-
Phase III trials involve thousands of participants across multiple global sites, requiring extensive coordination and infrastructure. The complexity includes long patient follow-up periods, rigorous data collection, monitoring for adverse events, and compliance with diverse regulatory requirements. Operational costs cover staffing, logistics, site management, patient recruitment, and advanced data management systems. These factors collectively make Phase III the most resource-intensive and costly phase in drug development.
-
Randomization ensures that participants are assigned to treatment or control groups without bias, distributing known and unknown confounding variables evenly. This prevents selection bias and supports the reliability of comparisons. Blinding—whether single or double—conceals group assignments from participants and/or investigators, minimizing performance and assessment biases. These design elements work together to maintain the objectivity of results, strengthening the credibility and regulatory acceptance of the trial data.
-
Regulatory agencies such as the FDA (United States) and EMA (Europe) establish stringent guidelines governing trial design, conduct, data integrity, and patient safety. They review trial protocols, monitor ongoing compliance, and assess final data to ensure scientific validity and ethical standards. Agencies enforce regulations like FDA’s 21 CFR Parts 312 & 314 and ICH-GCP principles, which safeguard participant welfare and ensure that trial outcomes are reliable enough to support drug approval and market entry.
-
Effective patient recruitment is critical and often challenging due to strict eligibility criteria and the need for diverse populations. Strategies include targeted digital outreach campaigns, partnerships with patient advocacy groups, and educational programs to raise awareness. Simplifying the informed consent process and clearly communicating the trial’s benefits and risks build trust and willingness to participate. Additionally, leveraging electronic health records and registries can identify potential participants faster, reducing enrollment timelines and improving trial success rates.