Fundamentals of Clinical Trials and Phases of Clinical Trials
Clinical trials are the backbone of medical research, providing invaluable insights into the effectiveness and safety of new treatments. Understanding the fundamentals of clinical trials and the clinical trial phases is crucial for anyone involved in clinical research. This comprehensive guide will explore the basics of clinical trials, including clinical trial phases, the clinical trials process, and how to stay on top of industry changes in 2025 with CCRPS.
A Blueprint for Reliable Research
Fundamentals of Clinical Trials :
Most errors in clinical trials are from poor planning. A good clinical trials process has to be built on solid research objectives. Clinical trial fundamentals training is important to assist researchers in designing their studies properly. The purpose of clinical trials is to determine the effects of a medical intervention. Other important features of a clinical trial that enable the attainment of this objective include: randomization, blinding, prospective evaluation, and the use of a control group.
Here are some fundamental issues to consider when designing clinical trials:
Fig 1: Clinical Trial ICH GCP
• Clearly defining the research question
• Minimizing variation
• Randomization
• Stratification
• Selection of the population for trial
• placebo
• The selection of endpoint
• Sample size
• Planning of internment analysis
Phases of Clinical Trials
Fig 2: Fundamentals of clinical trials
Every clinical trial is designed based on basic research questions. These research questions must be clear enough that they can take a vague concept and make it into a specific hypothesis that we can try to support or fail. There are two methods of designing research questions. One approach is hypothesis testing in which research submits a thanks hypothesis and then poses questions to negate it. On the other hand, there is an alternative hypothesis that consists of questions supporting it. The second strategy is estimation. For instance, a trial has to be conducted to determine the extent of the difference between two therapies with optimal accuracy. (Scott R. Evans, 2011)
What is a randomized clinical trial?
Randomization is a very powerful way of preventing bias in clinical trials. In randomized clinical trials, the subjects were allocated randomly to one or more than two therapies and then treated identically, identical potential visible. Randomized control trials are the most critical way of determining whether a cause-effect relationship exists between treatment and outcome and for accessing the cost-effectiveness of treatment.
Clinical trials are divided into phases based on the object of the trial. There are four phases of clinical trials that are as follows.
1-Phase 1 clinical trial
2- Phase 2 clinical trial
3- Phase 3 clinical trial
4- Phase 4 clinical trial
What is a phase 1 clinical trial?
Phase 1 trials, also called drug escalation in human pharmacology, are the first time a new drug is taken by humans. These studies include a small number of healthy individuals or patients and are designed to determine the safety of a drug and find the right dose that will not cause toxicity.
What is a phase 2 clinical trial?
The second phase of the clinical trial is called therapeutic exploratory and is conducted with a second, larger group of people with the disease of interest. They serve to determine the safety, pharmacokinetics, and pharmacodynamics of the drug. Phase 2 is important in determining if the drug has a potential to move to the larger, more complex Phase 3 trials.
What is a phase 3 clinical trial?
In Phase 3, also known as therapeutic confirmatory, participants are more diverse. They determine the drug’s effectiveness in a wider population and watch for normal side effects. Phase 3 trials are meant to produce the information that is required for FDA approval and to take the drug to the market.
What is a phase 4 clinical trial?
Phase 4 trials, also referred to as therapeutic use, are done after the drug has gotten FDA approval. These trials are also observational and are used to find less common adverse effects and to confirm the long term efficacy of the drug in a real world setting.
Fig 3: Phases of Clinical Trials
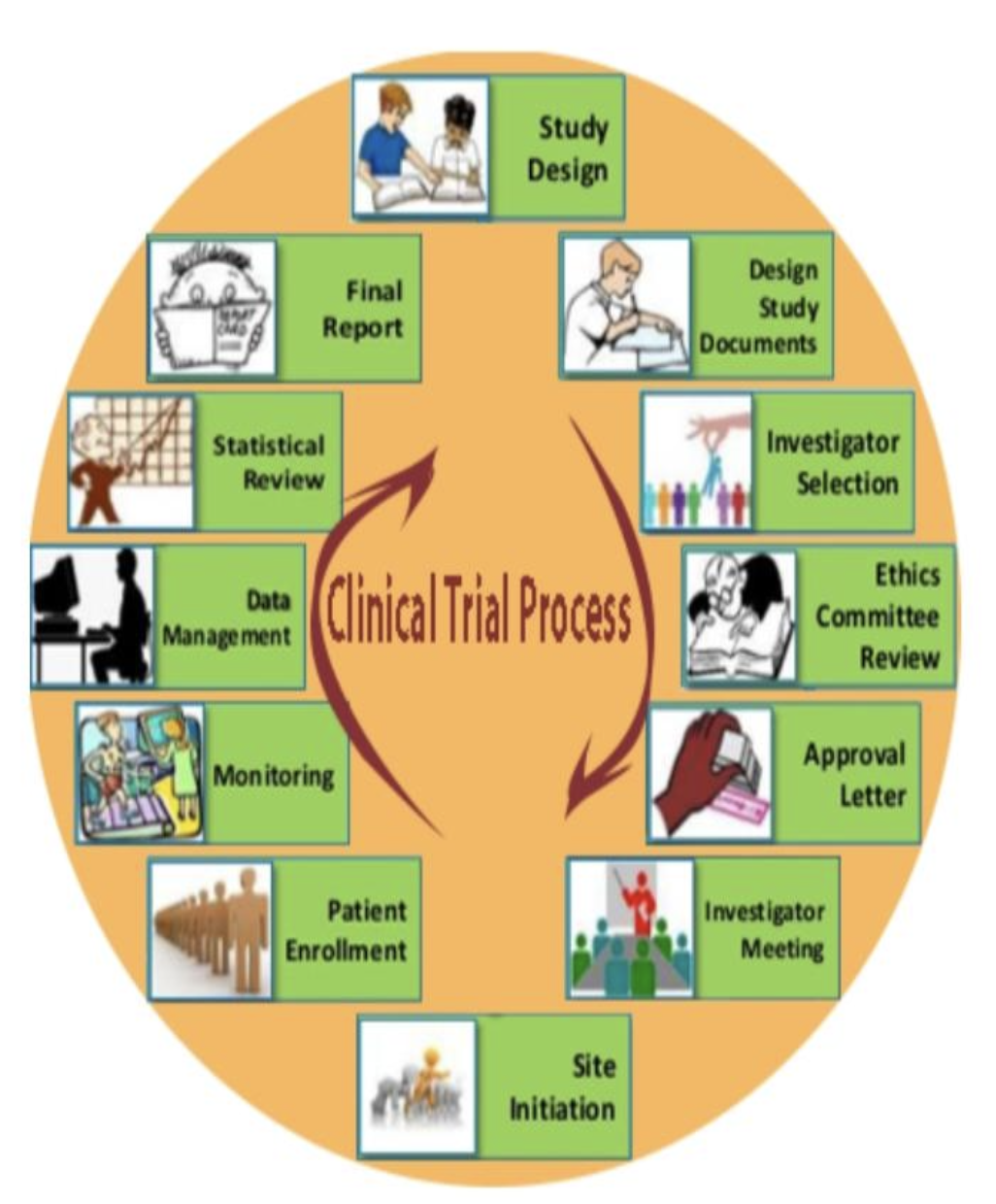
Clinical Trial Process: A Roadmap to Success
The clinical trial process covers patient recruitment and post-trial monitoring as well as the research and design phase of the study. These steps should be followed to ensure that the trials are conducted efficiently and ethically as well as within regulatory guidelines.
Key steps in the process include:
Study Design: A well-defined research question and hypothesis.
Ethics Approval: Obtain ethical clearance from regulatory bodies.
Patient Recruitment: Ensuring participants are properly selected and informed.
Data Collection: Gathering and analyzing data according to the trial design.
Monitoring and Reporting: Ongoing oversight to ensure safety and compliance.
To be successful, clinical trials have to be planned carefully, and a Clinical Trial Management System (CTMS) can be useful in that effort. Check out our guide on Clinical Trial Management System to explore the essential features that can streamline your clinical trials in 2025.
The Importance of Clinical Trial Fundamentals Training in 2025
As clinical trials become increasingly complex, clinical trial fundamentals training is more important. Clinical trial basics and the clinical trial phases basics: Stay current on the fundamentals of clinical trials and help researchers make informed decisions and avoid costly mistakes. If you’re aiming for a career in clinical research, our Guide on Becoming a CRA in 2025 is a valuable resource for those interested in becoming a Clinical Research Associate (CRA).
Explore Courses for Clinical Research Career
Courses Available:
Conclusion
In conclusion, mastering the fundamentals and phases of clinical trials is essential for advancing medical research and bringing innovative treatments to market. With a solid understanding of these processes, researchers and clinical professionals can enhance the effectiveness and safety of medical interventions. For those looking to deepen their expertise, CCRPS provides comprehensive resources and training to stay ahead in the evolving field of clinical research.
References:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3083073/ - Fundamentals of clinical trial design
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3272827/ - Key Concepts of Clinical Trials: A Narrative Review
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5989153/ - Clinical trial design and dissemination: comprehensive analysis of clinicaltrials.gov and PubMed data since 2005
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1884542/ - What is a clinical trial?
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6662388/ - Regulatory Affairs 101: Introduction to Investigational New Drug Applications and Clinical Trial Applications