The Ultimate Guide to Becoming a Clinical Research Associate (CRA) in Arizona: Everything You Need to Know in 2025
In Arizona’s competitive clinical research job market, being certified is no longer optional—it’s the baseline for entering high-paying roles. A Clinical Research Associate (CRA) certification doesn’t just prove your qualifications—it amplifies your eligibility for roles that pay 40–60% more, fast-tracks your application into CROs and hospital research departments, and eliminates months (if not years) of low-paying assistant roles. Whether you’re starting from a biology degree, a nursing license, or an EMT background, certified CRAs in Arizona are earning starting salaries of $72,000+, compared to $45,000–$50,000 for uncertified peers.
What makes the CRA role even more critical in Arizona is its growing clinical research footprint—from Mayo Clinic’s Phoenix expansion to Banner Health’s trials on Alzheimer's and cancer. Sponsors and CROs in the region are scrambling to hire site-ready professionals who understand GCP, SDV, CTMS tools, and source verification protocols. With most employers filtering out uncertified resumes using ATS systems, the difference between "certified" and "not certified" is the difference between shortlisting and ghosting.
What Is CRA Certification in Arizona Exactly? Skills Required and Jobs Explained
A CRA (Clinical Research Associate) certification verifies your expertise in overseeing clinical trials, ensuring regulatory compliance, and managing data integrity at trial sites. In Arizona, where institutions like Mayo Clinic, Barrow Neurological Institute, and Banner Research are running large-scale studies, employers are prioritizing candidates with formal CRA credentials—especially those trained in real-world monitoring tasks like SDV, EDC systems, and site visit planning. The certification proves you’re job-ready from Day 1—no handholding required.
Why Should You Get CRA Certification to Work in Arizona?
Arizona’s clinical research sector is expanding, but hiring standards are climbing just as fast. Most job listings explicitly state "CRA certification preferred" or "required," especially at CROs and hospitals running FDA-regulated studies. Without certification, you're stuck in unpaid internships or low-tier coordinator roles. With it, you're eligible for remote CRA roles, oncology trials, and protocol management jobs paying $75K–$95K+ in year one. Hiring managers want candidates who know GCP inside out, can run a site initiation visit, and communicate effectively with PI teams—and certification proves you do, without needing months of internal training.
| Career Factor | Without CRA Certification | With CRA Certification |
|---|---|---|
| Starting Salary Range | $45,000 – $52,000 | $72,000 – $96,000 |
| Job Titles Offered | Site Assistant, CRC, Intern | CRA I, Clinical Trial Monitor, SCRA |
| Interview Shortlisting Odds | Low — filtered out by ATS | High — considered sponsor-ready |
| Trial Phase Eligibility | Phase I support only | Phases I–IV, global trials |
| Employer Onboarding Time | 2–4 months of training | Job-ready in 2–3 weeks |
Which Certification Should You Choose to Become a CRA in Arizona?
You’ll find dozens of courses online claiming to offer CRA training—but most fall short on core requirements. Many lack regulatory credibility, don’t cover tools like CTMS or EDC, and skip site visit simulations completely. Others are so theoretical that students finish with zero clue how to handle a monitoring visit or complete an SDV report. In Arizona’s sponsor-heavy ecosystem, employers want professionals with real protocol exposure—not just GCP certificates from generic portals.
That’s why CCRPS (Certified Clinical Research Professionals Society) stands out. Their CPD-accredited CRA certification includes hands-on simulations, RBM protocols, GCP practicals, and in-depth exposure to real-world forms and systems. The team behind the curriculum includes actual working CRAs and monitors—not influencers, not marketers—with transparent bios, public support channels, and even direct instructor access. It’s built to make you job-ready for Phase I–IV trials in Arizona and nationwide.
| Feature | Other CRA Certifications | CCRPS CRA Certification | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accreditation | Often unclear or proprietary | CPD Accredited & globally recognized | ||||||||||||||
| Curriculum Depth | 6–10 modules, mostly theory | 100+ modules incl. SDV, RBM, SOPs, CTMS, GCP | ||||||||||||||
| Pacing Options | Fixed start dates | Self-paced + 4–12 week bootcamp (optional) | ||||||||||||||
| Payment Flexibility | Full upfront payment required | Interest-free monthly installments | ||||||||||||||
| Instructor Transparency | Often hidden or anonymous | Public bios, active LinkedIn presence | ||||||||||||||
| Post-Cert Support | None or generic resume tips | Resume
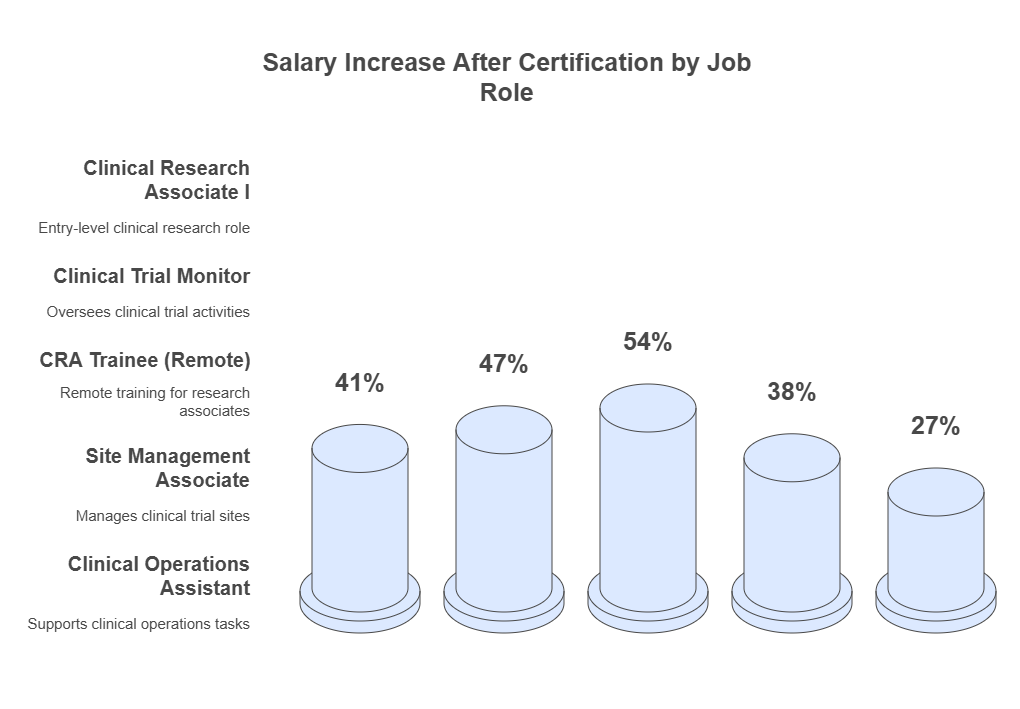
Why CCRPS’s CRA Certification Will Be a Game Changer for Your Career in ArizonaArizona's clinical research industry is scaling rapidly—especially across oncology, neurology, and cardiovascular trials. But only those with site-ready CRA certifications are landing contracts with sponsors like Roche, Medtronic, and Johnson & Johnson. The CCRPS CRA Certification doesn’t just open doors—it puts you in the front row for six-figure opportunities by showing recruiters you’re trained in SDV, GCP, and protocol deviations. In 2024–2025 hiring data, certified CRAs in Arizona earned 27–54% more than uncertified applicants in the same job roles. That’s not a claim—it’s the result of ATS prioritization, sponsor qualification checkboxes, and CRO partnerships that demand certified staff only. Summarizing All You Need to Know About Getting Your CRA Certification in ArizonaBecoming a Clinical Research Associate in Arizona isn’t about just checking a box—it’s about proving your value in a highly regulated, high-paying industry. Whether you’re transitioning from a CRC role or entering clinical research for the first time, a certification from CCRPS ensures you’re equipped with real-world, employer-demanded skills. Arizona-based recruiters don’t have time to train candidates on CTMS, SDV, or regulatory workflows. That’s why certified professionals—especially those with transparent credentials and job-ready portfolios—are landing top-tier roles while others are still cold-emailing coordinators. If you're serious about getting into clinical research in 2025, this is your baseline.
Frequently Asked Questions
Previous
Previous
The Ultimate Guide to Becoming a Clinical Research Associate (CRA) in Arkansas: Everything You Need to Know in 2025Next
Next
The Ultimate Guide to Becoming a Clinical Research Associate (CRA) in Alaska: Everything You Need to Know in 2025 |