Top 5 Clinical Trial Data Management Strategies You Should Know
In the rapidly evolving landscape of clinical research, effective data management is paramount. As we step into 2025, the integration of advanced technologies and methodologies has transformed how clinical trials are conducted. This article delves into the top five clinical trial data management strategies that are shaping the future of clinical research.
1. Data Standardization
Data standardization in clinical trials means organizing and formatting data consistently across all studies, platforms, and systems. It ensures that clinical data collected from various sources (like patient forms, lab results, and devices) are compatible and can be interpreted uniformly.
Standardization usually follows global regulatory frameworks like:
CDISC (Clinical Data Interchange Standards Consortium)
HL7 (Health Level Seven International)
These standards define how data should be structured and coded (for example, using the same labels for things like “blood pressure” across studies).
Importance of Data Standardization
Enhanced Data Quality: Standardized data reduces errors and discrepancies, leading to more reliable results.
Facilitated Data Sharing: Uniform data formats make it easier to share and compare data across studies and institutions.
Regulatory Compliance: Regulatory bodies often require standardized data for submissions, ensuring smoother approval processes.
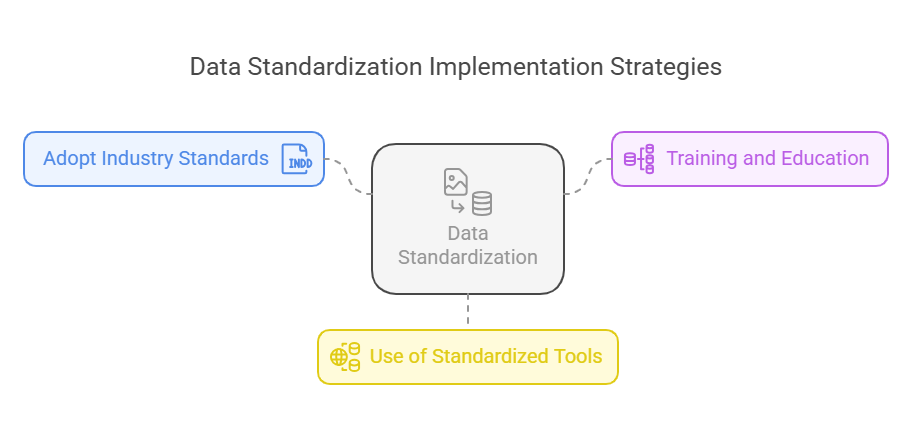
Implementation Strategies
Adopt Industry Standards: Utilize established standards like CDISC (Clinical Data Interchange Standards Consortium) for data formatting.
Training and Education: Ensure that all team members are trained in data standardization practices.
Use of Standardized Tools: Implement software solutions that enforce data standardization protocols.
Related Blog: Why Clinical Trial Management is Essential for Pharma Companies?
2. Use of Electronic Data Capture (EDC)
Electronic Data Capture (EDC) is a software system used to collect, manage, and store clinical trial data digitally—replacing traditional paper-based methods.
Instead of researchers writing data on paper and later transcribing it, EDC platforms allow them to enter data directly into a secure, cloud-based system through laptops, tablets, or even mobile devices in real time.
Popular EDC systems include:
REDCap
Medidata Rave
OpenClinica
These platforms streamline data collection, improve accuracy, and support compliance with regulatory standards.
Benefits of EDC Systems
Improved Data Accuracy: Real-time data entry and validation checks reduce errors.
Enhanced Efficiency: Streamlined data collection accelerates the trial process.
Remote Accessibility: Authorized personnel can access data from anywhere, facilitating remote monitoring.
Cost Savings: Reduced need for physical storage and manual data handling lowers operational costs.
Best Practices for EDC Implementation
Stakeholder Engagement: Involve all relevant parties in the selection and implementation of EDC systems.
Comprehensive Training: Provide thorough training to ensure effective use of the system.
Continuous Monitoring: Regularly assess the system's performance and make necessary adjustments.
3. Centralized vs. Decentralized Data Management
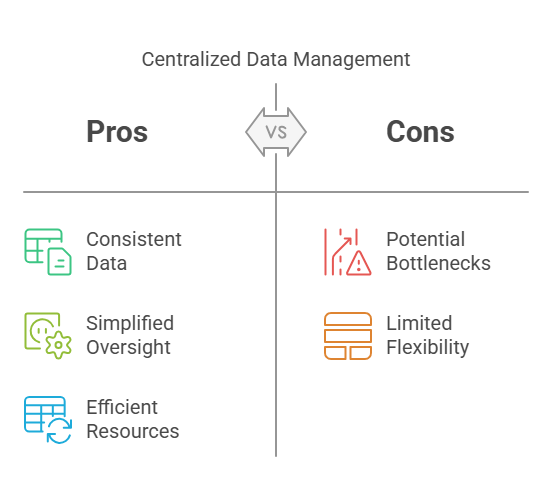
Centralized Data Management:
All data from different trial sites is collected and processed in a single, central system managed by one team. This setup provides uniformity and tight control.
Pros:
Consistent Data Handling: Uniform procedures across all sites.
Simplified Oversight: Easier to monitor and control data processes.
Efficient Resource Utilization: Centralized systems can be more cost-effective.
Cons:
Potential Bottlenecks: Central points of control may slow down processes.
Limited Flexibility: May not accommodate site-specific needs effectively.
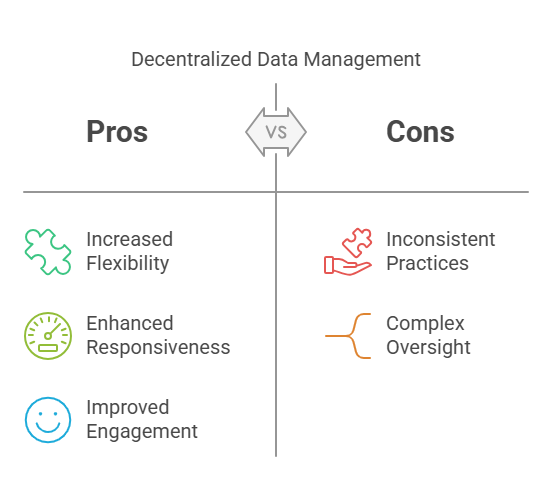
Decentralized Data Management:
Each research site (e.g., hospitals or clinics participating in the study) manages its own data locally. This method offers flexibility and quicker local decision-making.
Pros:
Increased Flexibility: Sites can tailor processes to their specific requirements.
Enhanced Responsiveness: Faster decision-making at the local level.
Improved Patient Engagement: Closer interaction with participants.
Cons:
Inconsistent Data Practices: Variability across sites can lead to data discrepancies.
Complex Oversight: Monitoring multiple systems can be challenging.
Many modern trials use hybrid models that combine the benefits of both systems.
Related Blog: The Role of Data Management in Successful Clinical Trials
4. Risk-Based Monitoring (RBM)
Risk-Based Monitoring is a method that prioritizes the monitoring of data and activities that are most critical to patient safety and data quality.
Instead of monitoring 100% of all collected data (which is time-consuming and costly), RBM focuses on key risk indicators using:
Central monitoring dashboards
Trigger alerts
Statistical algorithms
Regulators like the FDA and EMA have endorsed RBM as a smarter, more efficient way to oversee clinical trials.
Advantages of RBM
Targeted Monitoring: Allocates resources to high-risk areas, improving oversight.
Cost Efficiency: Reduces unnecessary site visits and monitoring activities.
Enhanced Data Integrity: Focuses on critical data points, ensuring their accuracy.
Regulatory Support: Endorsed by agencies like the FDA and EMA for its effectiveness.
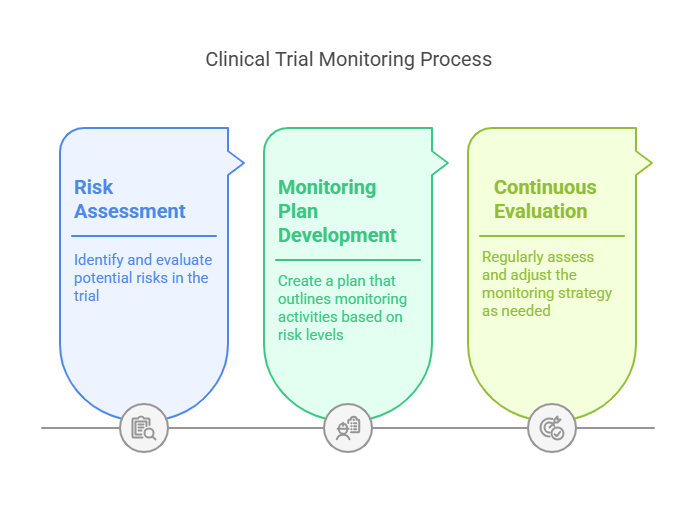
Implementation Steps
Risk Assessment: Identify and evaluate potential risks in the trial.
Monitoring Plan Development: Create a plan that outlines monitoring activities based on risk levels.
Continuous Evaluation: Regularly assess and adjust the monitoring strategy as needed.
5. Integration with Other Systems
Integration means connecting the clinical data management system (CDMS) with other digital tools involved in a clinical trial, such as:
CTMS (Clinical Trial Management System)
EHR (Electronic Health Records)
ePRO (Electronic Patient-Reported Outcomes)
IVRS (Interactive Voice Response Systems)
This seamless flow of data ensures that information is shared automatically across systems, reducing the need for manual entry and enabling real-time decision-making.
Benefits of System Integration
Streamlined Workflows: Automated data transfer reduces manual entry and errors.
Real-Time Data Access: Immediate availability of data supports timely decision-making.
Improved Collaboration: Facilitates communication among different departments and stakeholders.
Regulatory Compliance: Integrated systems often include features that support adherence to regulatory requirements.
Integration Strategies
Assess Compatibility: Ensure that systems can effectively communicate and share data.
Data Mapping: Define how data fields correspond between systems.
Testing and Validation: Conduct thorough testing to confirm accurate data transfer and functionality.
Related Blog: How to Choose the Right Clinical Trial Management Software?
10 Lesser-Known Facts About Clinical Trial Data Management
1. Data Standardization Reduces Trial Start-Up Time by Up to 30%: When sponsors use CDISC-compliant data formats from the beginning, they often shave weeks off the setup phase of a trial due to pre-configured forms and templates. (Source)
2. EDC Systems Can Predict Query Volume Using AI: Modern EDC platforms use machine learning algorithms to forecast how many data queries might arise during a trial—helping teams plan staffing and timelines. (Source)
3. Hybrid Data Management Models Are on the Rise: As of 2025, more than 60% of sponsors use a hybrid approach (part centralized, part decentralized), especially in global trials where site flexibility is essential. (Source)
4. Blockchain Is Being Piloted for Immutable Audit Trails: Some Phase I and II trials are experimenting with blockchain to create tamper-proof data logs for regulatory audit readiness. (Source)
5. RBM Can Reduce Monitoring Costs by 20-40%: By focusing on critical risk indicators, RBM reduces unnecessary site visits and data checks—significantly lowering operational costs. (Source)
6. Integration with Wearable Tech Is Becoming Standard: Wearables (like Fitbit or Apple Watch) are now integrated with EDC and ePRO tools, enabling real-time vitals tracking directly into the trial database.
7. EDC Logs Are Legally Auditable for Up to 25 Years: Regulations in the EU and US require that all data, including EDC logs and system access history, be kept for decades post-trial.
8. RBM Can Detect Fraud Through Statistical Anomalies: Central monitoring tools can flag unusual patterns in site data entry—often indicating fabrication or fraud.
9. Real-Time Dashboards Are Reducing Data Lag by 80%: Sites with integrated dashboards report almost real-time data visibility, drastically cutting down on reporting delays.
10. Clinical Data Managers Are Among the Fastest-Growing Roles in Healthcare Tech: As trials grow more digital, demand for skilled data managers is projected to grow by 22% from 2024 to 2029.
Explore Courses for Clinical Research Career
Courses Available:
Conclusion
Effective clinical trial data management is essential for ensuring data quality, regulatory compliance, and patient safety. By understanding and implementing strategies like data standardization, electronic data capture, centralized or decentralized models, risk-based monitoring, and system integration, clinical research teams can streamline operations and improve trial outcomes in 2025 and beyond.
As the industry continues to evolve with AI, wearable tech, and smarter monitoring tools, staying informed and proactive is key. At CCRPS, we are committed to equipping professionals with the latest training and certifications in clinical research data management to help you stay ahead in this dynamic field.
Frequently Asked Questions (FAQs)
-
The most widely used data standard is CDISC (Clinical Data Interchange Standards Consortium), particularly the SDTM (Study Data Tabulation Model), which is required by the FDA and other regulatory bodies for submissions.
-
Modern EDC systems use end-to-end encryption, multi-factor authentication, and compliance with 21 CFR Part 11 and GDPR standards, making them highly secure for storing sensitive patient data.
-
Centralized trials gather all data in one location and are easier to control.
Decentralized trials allow data collection from remote or local sites, offering more flexibility—especially for virtual or hybrid trials.
-
While not mandatory, RBM is highly recommended by regulatory authorities like the FDA and EMA. It is increasingly adopted to optimize monitoring resources, especially in large-scale or global trials.
-
By integrating systems like EDC, CTMS, and ePRO, clinical trials reduce manual data entry, eliminate duplication, and enable real-time data flow, making trial management more seamless and efficient.