Your Complete Guide to Clinical Project Management Course in New York: Everything You Need to Know in 2025-2025
New York is the nerve center of global clinical trials—home to top-tier academic hospitals, biotech startups, and sponsor-driven studies. But the era of climbing from CRA to manager through years of passive experience is over. Today, clinical project leadership demands strategic, certified professionals who can manage timelines, vendors, and regulatory compliance across multi-million-dollar trials. Whether you’re in NYC, Long Island, or Westchester, the demand for certified Clinical Project Managers is surging—and the gap between trained and untrained talent is growing wider every quarter.
With a recognized Clinical Project Management certification, you can leapfrog into roles that not only offer 30–60% higher salaries but also position you for global trial coordination, inspection readiness, and team leadership. In New York’s fast-paced, compliance-heavy research environment, certification isn't just a boost—it’s the standard that gets you hired faster, paid more, and promoted quicker.
What Is Clinical Project Management Certification in New York? Skills Required and Jobs Explained
A Clinical Project Management (CPM) certification in New York equips professionals to lead cross-functional clinical trials—not just coordinate isolated tasks. Unlike general project management training, this certification is built around GCP compliance, stakeholder oversight, trial budgeting, regulatory frameworks (FDA, ICH-GCP, CFR Part 11), and team communication across multi-site U.S. trials. In New York’s trial-heavy environment—where academic institutions, sponsor companies, and CROs constantly launch new studies—certification isn’t optional anymore, especially for mid-level professionals eyeing leadership.
What Skills Does a Certified Clinical Project Manager in New York Actually Gain?
FDA-centered regulatory document workflows (IND, 1572, ICF, TMF structure)
Budget forecasting, vendor oversight, and milestone-based contract structuring
Timeline enforcement, risk-based monitoring (RBM), and deviation handling
Protocol design support, stakeholder communication, and CRA team leadership
SAE follow-up workflows, AE trend tracking, and MedDRA/CTCAE familiarity
Clinical systems mastery (CTMS, eTMF, EDC platforms like Medidata or Veeva)
Inspection readiness (FDA, sponsor, IRB), CAPA documentation, and GCP audits
Real-world experience through project simulations and downloadable execution templates
What Jobs Can You Apply for With Clinical Project Management Certifiication in New York?
Clinical Project Manager (CPM) (Pfizer NYC, Regeneron, BMS New Brunswick)
Trial Start-Up Manager (Columbia University Research Center, Parexel Manhattan)
Clinical Operations Manager (Weill Cornell, Flatiron Health, ICON)
Regional Clinical Trial Lead (Moderna, Roche NYC, IQVIA East Coast)
Clinical Risk & Quality Manager (Memorial Sloan Kettering, Veristat, Labcorp)
Regulatory Program Manager (NYU Langone, Mount Sinai, FDA-linked research units)
Why Should You Get Clinical Project Management Certification to Work in New York?
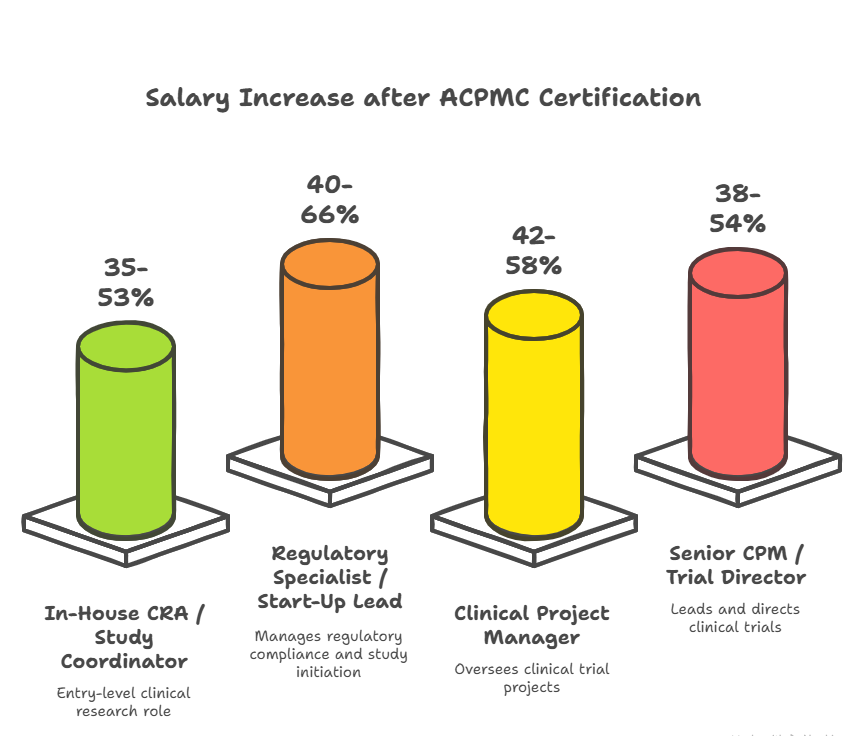
New York is home to some of the most competitive, compliance-heavy clinical research environments in the world. From Columbia to Pfizer to Mount Sinai, employers no longer accept “learn-as-you-go” management. They expect Clinical Project Managers to be GCP-proficient, systems-literate, audit-ready, and capable of running multi-site budgets, timelines, and teams from day one. Without certification, professionals are often locked into support roles—admin-heavy, CRA-assisting, and sponsor-removed. With certification, you step into full project ownership and become the central point of execution, oversight, and escalation.
In New York, this shift can mean $30K–$60K/year in salary difference, faster promotions, and eligibility for leadership-track roles within CROs, sponsors, and IRB-regulated institutions.
| Career Element | Without Certification | With Clinical Project Management Certification |
|---|---|---|
| Job Title | In-house CRA / Study Coordinator | Clinical Project Manager / Trial Lead |
| Hiring Priority | Low – not sponsor-facing or leadership-ready | High – trusted to lead timelines and teams |
| Salary Range (NY Avg) | $65,000 – $88,000/year | $105,000 – $145,000/year |
| Career Growth Timeline | 5+ years to reach management | 12–24 months to team or regional lead roles |
| Trial Ownership | Limited to support and data cleanup | Full control of protocol, timeline, and team deliverables |
| Audit & Sponsor Trust | Viewed as junior/support tier | Eligible for inspection lead, budget escalations, and cross-functional oversight |
Which Certification Should You Choose to Become a Clinical Project Manager in New York?
In New York’s competitive clinical research market, dozens of programs promise “project management” credentials—yet most are generic, theoretical, or geared toward construction or IT workflows. Short courses from platforms like Coursera or university extensions offer PMBOK or GCP overviews but skip over budget controls, RBM implementation, CRO oversight, or inspection readiness. And while some may award certificates, they rarely include real-world tools, career mentorship, or trial simulation experience.
That’s where the CCRPS Advanced Clinical Project Management Certification (ACPMC) stands alone. Designed for CRAs, CRCs, MSLs, and clinical operations professionals, ACPMC offers 284 modules, delivered 100% online and self-paced, plus weekly live mentoring and lifetime access. Jointly accredited (CPD, AMA, ANCC, ACPE) and eligible for 17.5 CME credits, this program covers everything from decentralized trials and AI integration to CTMS mastery, CRO negotiation, protocol design, and FDA inspection readiness. Graduates report $30K–$60K salary jumps, with job titles like Trial Director and Clinical Program Lead.
| Feature | Other Certifications | CCRPS ACPMC Certification |
|---|---|---|
| Accreditation | Often non-clinical or unclear | Jointly accredited: CPD, AMA, ANCC, ACPE (17.5 CME credits) |
| Curriculum Depth | 10–30 lessons, general PM focus | 284 modules covering regulatory ops, AI, DCTs, protocol design |
| Format | Self-paced or rigid schedules, no mentorship | 100% online, self-paced + live weekly webinars |
| Real-World Tools | Minimal or template-free | Downloadable TMF templates, KPI dashboards, trial budgets, audit checklists |
| Instructor Transparency | Often anonymous or pre-recorded only | Built by senior CRAs/PMs, with direct mentor interaction |
| Assessment | Quizzes or none | Proctored exam, validated certification, LinkedIn badge |
| Career Outcomes | Limited job impact or unclear pathways | Reported $30K–$60K increases, roles at Teleflex, ION, Inventprise |
| Refund & Flexibility | No refunds or limited trial | 14-day money-back guarantee, lifetime access, flexible payments |
Why CCRPS’s Advanced Clinical Project Management Certification Will Be a Game Changer for Your Career in New York
In New York, the average CRA or site coordinator spends 4–6 years trying to break into project leadership, often facing internal bottlenecks, credential barriers, and sponsor-side preference for certified talent. But CCRPS’s Advanced Clinical Project Management Certification (ACPMC) changes that equation. Graduates are entering mid-to-senior-level roles directly, thanks to deep hands-on training in protocol design, CRO management, digital systems, budgeting, and FDA-compliance workflows.
Certified professionals are getting fast-tracked by employers like Pfizer, Mount Sinai, Regeneron, and Moderna, while reporting $30K–$60K+ salary jumps and job mobility across biotechs, hospitals, and CROs. The certification doesn’t just teach theory—it builds real leadership execution across decentralized and global trials, including tools, simulations, and stakeholder strategy templates.
Summarizing All You Need to Know About Getting Your Clinical Project Management Certification in New York
Whether you're working at a hospital, CRO, sponsor, or research institute, the CCRPS Advanced Clinical Project Management Certification (ACPMC) is the most targeted and trusted pathway to transition into senior clinical project roles. With global accreditation, job-ready tools, and flexible learning, this certification delivers more than a credential—it delivers career leverage.
| Aspect | Details |
|---|---|
| Certification Name | CCRPS Advanced Clinical Project Management Certification (ACPMC) |
| Target Audience | CRAs, CRCs, MSLs, Research Associates, Trial Coordinators, Regulatory Professionals |
| Delivery Format | 100% online, self-paced + live weekly webinars |
| Accreditation | CPD, AMA, ANCC, ACPE — 17.5 CME credits |
| Curriculum Depth | 284 modules covering protocol, budgeting, digital trials, CRO oversight, and risk management |
| Included Tools | TMF templates, trial budget calculators, KPI dashboards, recruitment kits, risk logs |
| Final Assessment | Proctored certification exam + LinkedIn badge + instant PDF certificate |
| Career Outcomes | Clinical Project Manager, Program Lead, Trial Director, Regional Study Manager |
| Reported Salary Impact (New York) | $30K–$60K/year increase depending on role and seniority |
| Refund & Access | 14-day money-back guarantee + lifetime access + interest-free payment plans |
Frequently Asked Questions
-
No. The ACPMC certification is designed for clinical professionals transitioning into leadership roles, whether you’re a CRA, CRC, or even an MSL. You’ll start with foundational concepts like GCP-linked trial planning and regulatory compliance, then advance into high-level modules covering stakeholder management, CTMS systems, trial budgeting, and CRO/vendor oversight. Even if you’ve never led a protocol before, you’ll finish the course with the tools, simulations, and confidence to manage full trials. ACPMC prepares you for sponsor-facing roles with real-world execution—not just theory.
-
Yes. The ACPMC certification is jointly accredited by CPD, AMA, ANCC, and ACPE, and qualifies for 17.5 CME credits—making it highly respected across the U.S. In New York specifically, employers like Pfizer, Regeneron, Memorial Sloan Kettering, and IQVIA have hired graduates from CCRPS programs. Because it includes a proctored final exam, project simulations, and job-ready tools, it’s valued by CROs and research institutions as proof that you're ready to manage timelines, protocols, and cross-functional teams from day one.
-
Most learners complete the course in 6 to 8 weeks while working full-time. Since it’s 100% self-paced, you can move faster or slower based on your schedule. You’ll get lifetime access to all 284 modules, so you can revisit any content when needed. Weekly live webinars provide additional coaching and expert feedback. The course is structured into digestible lessons so you can progress efficiently—typically requiring around 40–60 hours total to complete, including the final proctored exam.
-
Unlike generic online courses, ACPMC includes a full library of real-world tools you can plug directly into your clinical workflows. These include trial budget calculators, TMF templates, protocol development frameworks, KPI dashboards, CRO oversight forms, and inspection readiness checklists. The tools are designed by practicing clinical project managers and are aligned with FDA and ICH-GCP requirements. These aren’t just downloads—they’re used in live trial environments and give you a competitive edge when applying or interviewing for CPM roles in New York.
-
Graduates have moved into titles like Clinical Project Manager, Research Program Lead, and Trial Director, often within 6–12 months. If you're earning $80K–$95K as a CRA or coordinator in NYC, completing ACPMC can position you for $120K–$160K+ roles depending on your experience. Employers value the certification because it demonstrates fluency in trial oversight, sponsor reporting, and risk mitigation. Alumni have secured roles at companies like Moderna, BMS, Emory, and Inventprise—many reporting salary jumps of $30K–$60K+ after certification.