Clinical Research Associate Exam Preparation Essential Study Guide
The demand for certified Clinical Research Associates (CRAs) is surging as clinical trials grow more complex and globally regulated. Companies now seek professionals who demonstrate regulatory expertise, monitoring precision, and exam-proven knowledge. The CRA exam is intentionally rigorous, designed to assess real-world application of Good Clinical Practice (GCP), ethical oversight, protocol compliance, and documentation accuracy—not just definitions or guidelines.
Many candidates fail not from lack of dedication, but from a disorganized approach to preparation. This guide breaks that pattern. You’ll learn how to master the exam structure, build competency across high-yield knowledge areas, and follow a milestone-driven study plan aligned with actual CRA responsibilities. Whether you're balancing work or studying full time, this resource gives you a roadmap to maximize retention, reduce overwhelm, and succeed on your first attempt. With the right method, tools, and mindset, certification becomes achievable—and career-defining.
Understanding the CRA Exam Structure
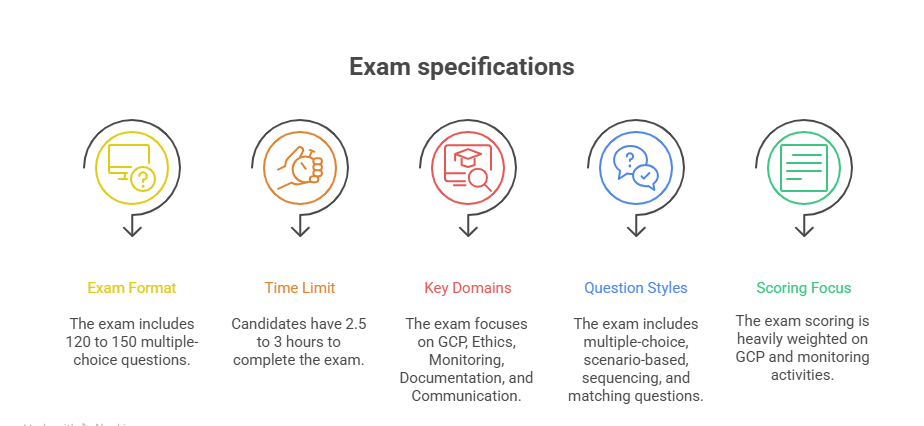
Preparing for the CRA exam begins with complete clarity on its structure. Many candidates dive into study materials without first understanding how the exam is built—and this is where most go wrong. To study effectively, you need to reverse-engineer your prep based on how the exam tests knowledge, judgment, and practical application. Below, we break it down by question format, domains covered, and the core topics that dominate the score weight.
Number of Questions, Format, and Domains
Most CRA certification exams include 120 to 150 multiple-choice questions, with some versions featuring scenario-based case studies. These aren’t recall questions—they assess how you would respond to real clinical research challenges using GCP, ICH, and regulatory logic.
Key domain breakdowns typically include:
Regulatory Guidelines (30–35%)
Monitoring & Site Management (20–25%)
Ethics & Human Subjects Protection (15–20%)
Clinical Operations & Trial Documentation (15–20%)
Communication & Professional Conduct (5–10%)
Expect strict time limits—most exams must be completed in 2.5 to 3 hours, meaning you’ll have roughly 60–90 seconds per question. This pace tests your ability to make decisions confidently under pressure.
The test format may also include:
Drag-and-drop sequencing (e.g., ordering monitoring visit steps)
Matching scenarios to GCP violations
Choosing the most appropriate document for a situation
GCP, Ethics, Trial Monitoring, and Documentation Focus
The exam heavily emphasizes GCP compliance and ethical conduct in human research. Expect questions that probe:
Informed consent procedures
Safety reporting timelines (SAEs, SUSARs)
Investigator responsibilities per ICH E6
Essential document maintenance (TMF, ISF)
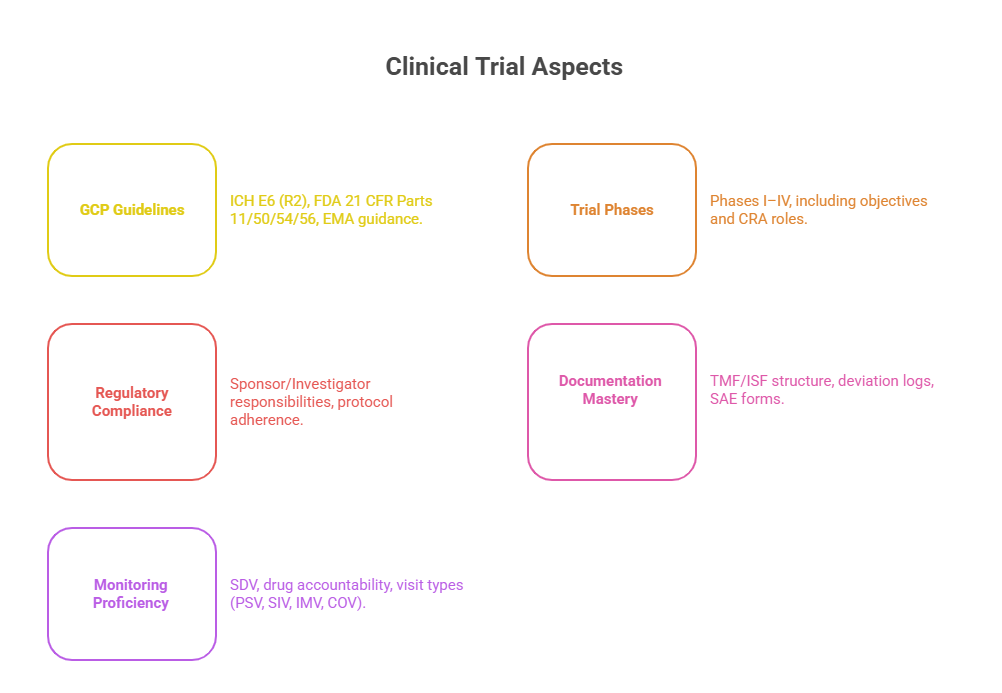
Monitoring-specific questions may ask how to handle protocol deviations, site noncompliance, or discrepancies in source data vs. CRFs. Documentation questions often focus on:
Audit trail expectations
Source data verification steps
Deviation logs and corrective action documentation
You’ll also encounter ethics-heavy case studies where you must determine what a CRA should do when patient welfare, data integrity, or site practices conflict with trial protocols.
Core Knowledge Areas to Master
Passing the CRA exam means more than reviewing slides and memorizing acronyms. You need deep functional knowledge across the entire clinical trial lifecycle. Every question on the exam is rooted in your understanding of how trials are governed, conducted, and monitored. Below, we break down the two most critical knowledge categories you must master to pass with confidence: regulatory frameworks and CRA-specific operational responsibilities.
Regulatory Guidelines (ICH-GCP, FDA, EMA)
You can’t pass the CRA exam without knowing regulatory guidelines inside out. The most heavily tested documents include:
ICH E6 (R2) – Defines the gold standard for Good Clinical Practice.
21 CFR Parts 11, 50, 54, 56 – Core FDA regulations on electronic records, informed consent, financial disclosure, and IRBs.
EMA GCP Guideline (EudraLex Volume 10) – Focuses on EU-specific compliance, including GDPR and trial approval pathways.
Expect the exam to probe:
What documents are “essential” under GCP
Timelines for SAE reporting under FDA vs EMA
Responsibilities of sponsors vs investigators
How to maintain audit readiness throughout the trial
You’ll also need clarity on clinical trial registration (e.g., ClinicalTrials.gov), informed consent version control, and differences between protocol amendments vs deviations.
Clinical Trial Phases and CRA Responsibilities
Understanding clinical trial phases is essential not just to define them, but to know what a CRA’s role is at each stage:
Phase I – Focus on safety, dose escalation, and PK/PD monitoring.
Phase II – Efficacy assessment and adverse event tracking.
Phase III – Large-scale verification of efficacy and long-term safety.
Phase IV – Post-marketing surveillance and real-world data collection.
Your CRA responsibilities across phases include:
Pre-Study Visits (PSV) – Site qualification, feasibility assessments.
Site Initiation Visits (SIV) – Training staff, confirming readiness.
Monitoring Visits – Source data verification (SDV), protocol compliance, drug accountability.
Close-Out Visits – Archiving documents, resolving pending issues, audit prep.
You’ll be tested on how to handle situations like:
A PI repeatedly missing AE documentation timelines
Inconsistent entries between source and eCRF
IRB approval delays or re-consent errors
Success comes from knowing how a CRA balances regulatory enforcement with site relationship management—and the exam reflects that dual responsibility.
Daily Study Schedule and Weekly Milestones
One of the biggest differentiators between candidates who pass and those who fail the CRA exam is study structure. High-performing test-takers don’t just “put in hours”—they use a milestone-driven schedule that targets retention, reinforcement, and application. Whether you're studying around a full-time job or have dedicated hours each day, the key is mapping your prep to a progressive learning framework that builds momentum weekly.
Ideal Time Allocation for Full-Time vs Part-Time Learners
Your study schedule depends heavily on your availability:
If you're studying part-time (1–2 hours/day):
Week 1–2: GCP, ICH E6 (R2), and 21 CFR Parts 11, 50, 54, 56
Week 3–4: CRA roles, trial phases, and monitoring practices
Week 5–6: Documentation, TMF, protocol deviations, SAE reporting
Week 7–8: Review, mock exams, and gap-filling
If you're studying full-time (4–6 hours/day):
Week 1: Deep-dive into regulations (GCP, FDA, EMA)
Week 2: Study trial operations, visit types, and role-play site scenarios
Week 3: Documentation drills, redacted audit reports, and deviation analysis
Week 4: Complete 3–4 timed practice tests + critical review of weak areas
Use time blocks, such as the Pomodoro technique, to prevent burnout and boost recall. For every 25 minutes of study, take a 5-minute break—and after 4 cycles, break for 30–45 minutes.
Built-In Review Days and Mock Test Strategy
Your prep must include built-in review cycles to avoid forgetting what you've covered. Every 6th or 7th day, block out 2–4 hours to revisit:
Previous weeks’ flashcards
Key regulatory notes
Questions you got wrong on practice exams
Mock test strategy:
Start mock testing by week 3 (not later)
Use 2–3 full-length exams from different providers
Simulate real conditions: no breaks, timed, quiet room
After each test, spend an entire study session reviewing why answers were wrong, not just what the correct answer is. This shifts your focus from memorization to judgment development—the same skill the CRA exam tests in case-based questions.
Finally, don’t stop revising even when you're confident. The exam rewards clarity, not overconfidence. The more consistent your schedule, the more exam-ready your instincts will become.
| Study Category | Details |
|---|---|
| Part-Time Learners | 6–8 weeks, 1–2 hours daily. Focus on building foundational understanding of ICH-GCP, regulatory frameworks, and trial operations. |
| Full-Time Learners | 3–4 weeks, 4–6 hours daily. Condensed plan covering all core knowledge domains, documentation, and exam practice. |
| Study Blocks | Use the Pomodoro method: 25 minutes of focused study followed by a 5-minute break. Take longer breaks every 4 cycles. |
| Mock Test Strategy | Begin mock testing by week 3. Take at least 3 full-length exams under timed conditions. Analyze errors after each session. |
| Review Days | Set aside 1 day per week to revisit flashcards, regulatory summaries, and weak points from prior sessions. |
Common Mistakes CRA Candidates Make
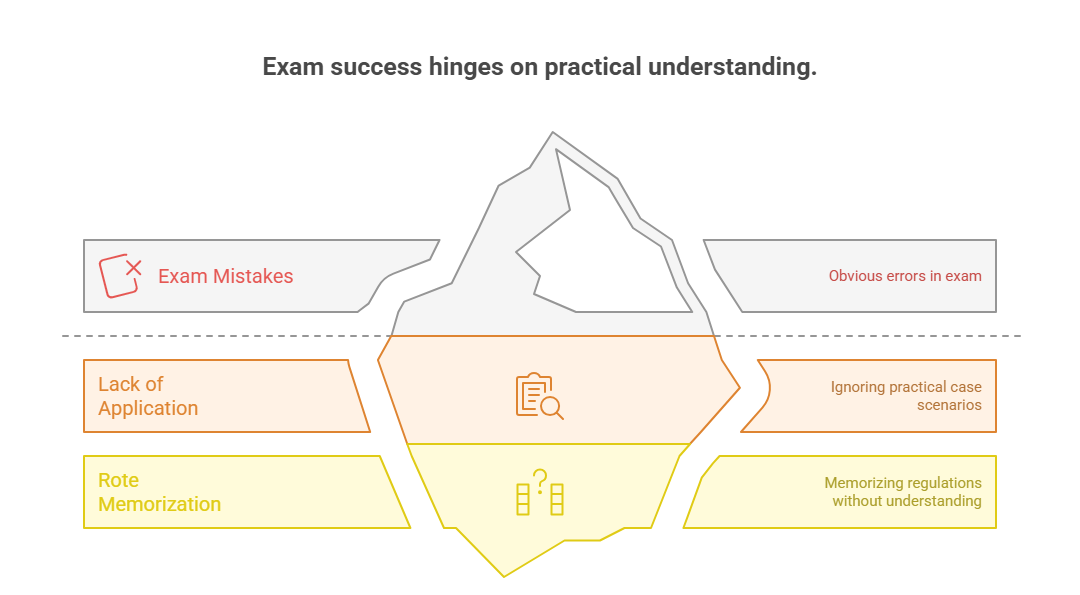
Even highly experienced professionals stumble on the CRA exam—not because they lack knowledge, but because they fall into avoidable traps. Understanding these mistakes is just as important as mastering the material. The exam isn't just about content; it tests your ability to apply guidelines under pressure, interpret gray areas, and spot compliance gaps in real-time situations. Below are the two most damaging mistakes—and how to avoid them.
Ignoring Practical Case Scenarios
Many candidates focus heavily on regulations but fail to prepare for real-world application. The CRA exam includes scenario-based questions where memorizing guidelines won’t help unless you can apply them. For example:
A question may describe a subject who was dosed before signed informed consent. Do you report it as a protocol deviation, serious breach, or both?
You might be asked how to handle a site where drug accountability logs are incomplete and no corrective action was taken.
To avoid this:
Use case simulation platforms and training modules that test situational judgment.
Practice translating GCP into actions, not just answers.
Discuss past trial challenges with certified CRAs or mentors to build real-world intuition.
Without that depth, you risk missing the point of 20–30% of the exam questions—especially those testing ethics, site management, and monitoring conduct.
Memorizing Instead of Applying GCP Principles
Another fatal error: trying to memorize line-by-line regulations rather than internalizing their purpose. You don’t need to recite ICH E6 verbatim—you need to understand why each clause exists and how it protects data integrity and subject safety.
For example:
It's not about knowing that the sponsor must provide the investigator brochure (IB)—it’s understanding that this document equips investigators with the information needed to ensure ethical dosing and risk management.
Instead of memorizing SAE definitions, understand what triggers expedited reporting and why timelines differ across FDA vs EMA.
To correct this:
Rewrite GCP sections in your own words to make sense of intent.
Link principles to site interactions—e.g., how you would educate staff during a Site Initiation Visit.
Take quizzes that ask "what should the CRA do next?" rather than just factual multiple-choice.
CRAs are held accountable not for rote recall, but for how they interpret, implement, and document decisions under pressure. Your exam results will reflect that.
Best Tools and Resources to Accelerate Learning
The difference between passing the CRA exam and falling short often comes down to which tools you use—and how you use them. Studying smart isn’t about piling on endless materials. It’s about choosing high-quality resources that mirror the exam’s logic, emphasize application, and fit your personal learning style. Below are the most powerful tools and resources top scorers use to prep effectively and efficiently.
Top-Rated Study Guides, Webinars, Flashcards
Not all guides are created equal. The best ones are designed specifically for CRA candidates and focus on exam-relevant case scenarios, audit readiness, and GCP comprehension. Top picks include:
ICH GCP E6 (R2) annotated guides – These explain not just what the clauses say, but how they apply to day-to-day CRA responsibilities.
Clinical research webinars – Live or recorded sessions by industry experts often cover real inspection outcomes, common site pitfalls, and regulatory updates.
Flashcard apps (Anki, Brainscape) – Ideal for drilling acronyms, regulatory deadlines, and visit procedures in short bursts. Look for CRA-specific decks, not general clinical research ones.
Bonus tip: print your flashcards physically and rotate them weekly, ranking them by confidence level. Active recall = deeper retention.
Question Banks, GCP Simulators, and Audit Templates
Practice questions are not just for testing—they're for training your thinking. A well-curated question bank mirrors the format, logic, and phrasing of the actual exam. Here’s what to prioritize:
Question banks with rationales – These help you understand why an answer is right or wrong, improving judgment rather than just memorization.
GCP simulators – Platforms that present simulated monitoring visits or protocol violations and ask you to choose the correct action. These are perfect for strengthening situational thinking.
Audit checklists and SOP templates – Reviewing redacted audit findings or sponsor SOPs will sharpen your sense of compliance. Questions about documentation errors or site CAPAs often relate directly to these.
Finally, join private CRA prep forums or LinkedIn groups where candidates share recent experiences, mock exam insights, and study schedules. The key is staying current, not studying in isolation.
Choose no more than 3–4 total tools and commit to mastering them. Switching between too many resources dilutes focus and wastes valuable time.
| Resource Type | Recommended Tools |
|---|---|
| Study Guides | ICH-GCP annotated guides, FDA/EMA regulatory primers, and CRA-specific textbooks like “The CRA’s Guide to Monitoring Clinical Research.” |
| Flashcards | Anki, Brainscape, or printed cards. Focus on regulatory acronyms, reporting timelines, and essential documents. |
| Webinars | Live or recorded sessions from CCRPS, ACRP, and DIA. Prioritize content focused on real audits and site visit practices. |
| Question Banks | Resources with rationale-based answers. Practice scenario-heavy MCQs with logic-based explanations (e.g., CCRPS, SOCRA-style banks). |
| GCP Simulators | Platforms like CCRPS’s simulation labs or CRO-based GCP modules. Focus on trial deviation management and SDV procedures. |
How CCRPS’ CRA Certification Training Ensures Exam Readiness
While self-study works for some, most CRA candidates benefit from structured, expert-led training—especially when it mirrors real-world responsibilities and the actual exam structure. The CCRPS Certified Clinical Research Associate (CRA) Training is built to do exactly that. It combines regulatory theory, protocol operations, and audit-based case simulations into a systemized course that fast-tracks exam readiness and builds career confidence.
288 Lessons with Live Webinars and Case Simulations
The CCRPS CRA certification includes 288 structured lessons covering ICH-GCP, FDA, EMA, clinical trial documentation, monitoring, and communication. But unlike typical video libraries, this program emphasizes:
Live webinars with instructors who explain how guidelines apply in sponsor-site interactions.
Case-based simulations that mimic CRA decision-making during protocol deviations, SAE reporting, and monitoring visits.
Downloadable SOPs and checklists used in real sponsor audits to help build documentation fluency.
Learners are guided through every core exam domain, with weekly check-ins, self-assessments, and optional mentorship add-ons. Whether you're a complete beginner or an experienced coordinator transitioning to a CRA role, the course provides a clear path to exam mastery.
Accredited by CPD, CME, ACCRE
Credibility matters—especially when your future employers or sponsors are reviewing your qualifications. The CCRPS CRA program is accredited by CPD, CME, and ACCRE, ensuring your training meets international clinical research standards.
Here’s why that matters for your exam prep:
CPD Accreditation confirms that your hours are counted as professional development and recognized globally.
CME Accreditation adds weight if you're in a clinical or healthcare-related field.
ACCRE Accreditation ensures compliance with academic standards for clinical research instruction.
More importantly, this triple accreditation gives you an edge on resumes, during site qualifications, and when sitting for employer-approved CRA exams.
Beyond the curriculum, CCRPS offers:
Mock exams with real-time feedback
Access to GCP simulation platforms
Discussion forums to review tricky concepts with peers and instructors
By aligning its course structure to the exam's knowledge domains and case-based question style, CCRPS doesn’t just prepare you to pass—it prepares you to excel.
Frequently Asked Questions
-
Most candidates require 6 to 8 weeks of focused preparation. If you’re studying part-time, aim for 1–2 hours daily, using a milestone-based plan that includes regulatory reading, mock exams, and review sessions. Full-time learners can compress prep into 3–4 weeks with 4–6 hours per day of structured study. The key is active learning, not passive review. Use flashcards, simulated GCP platforms, and timed mock tests to maximize retention. Starting with ICH-GCP E6 (R2) and building outward into trial phases, site visits, and documentation processes ensures that you're exam-ready and real-world capable.
-
The exam prioritizes regulatory compliance, ethical oversight, and trial monitoring accuracy. Focus areas include ICH-GCP, 21 CFR Parts 11, 50, 54, 56, SAE/SUSAR reporting, and CRA site visit procedures (PSV, SIV, IMV, COV). You’ll also see questions on trial phases, source data verification, informed consent management, and essential document maintenance. Many questions are scenario-based, requiring you to choose the best course of action in site-related dilemmas. Candidates who study using real-world audit findings and case simulations tend to outperform those who only memorize guidelines.
-
Most CRA certification exams do not require prior experience, but understanding the clinical trial process is essential. If you're new, consider completing a structured program like the CCRPS CRA Certification, which includes simulations, site visit breakdowns, and ICH-GCP alignment. These courses help bridge the gap for beginners and prepare you for both the exam and entry-level CRA roles. Having foundational knowledge of trial phases, regulatory documents, and monitoring responsibilities is more valuable than direct job experience when it comes to passing the exam.
-
Rather than memorizing verbatim text, focus on application-based understanding. Break down ICH-GCP into themes: subject protection, investigator responsibilities, sponsor duties, and documentation standards. Rewrite key points in your own words, and use flashcards or quizzes to test retention. Connect guidelines to real scenarios, such as handling consent errors, unreported AEs, or protocol deviations. Tools like annotated guides, regulatory flowcharts, and case-based Q&A drills help build intuitive knowledge. The exam rewards your ability to apply principles in context, not just repeat definitions.
-
At least 3 full-length mock exams are recommended. Start your first one by week 3 or 4 of study to identify knowledge gaps early. The goal is not just scoring high—but analyzing why you chose wrong answers and how to improve decision-making. Use question banks that offer rationale explanations, not just answer keys. Simulate exam conditions: timed, distraction-free, and without notes. Each mock should feel like the real thing—especially in pacing (60–90 seconds per question). The CCRPS CRA course includes mock exams modeled after actual industry test structures.
-
Passing scores vary by exam provider, but most require 70–75% correct answers. Since some questions carry more weight (e.g., regulatory vs general conduct), focus your prep on high-weighted domains like GCP compliance, monitoring, and documentation. Unlike academic exams, CRA tests are often competency-based, which means mastering a certain threshold of essential topics is critical. Make sure you review the scoring rubric or blueprint provided by your exam body. Practice exams with score tracking help you gauge readiness and adjust your study strategy accordingly.
-
Yes, CCRPS (Certified Clinical Research Professionals Society) is widely recognized and offers a globally accepted CRA certification. Their program is accredited by CPD, CME, and ACCRE, meeting rigorous international standards for clinical research education. It includes 288 lessons, live instructor-led webinars, case-based simulations, and downloadable monitoring tools. Employers, sponsors, and CROs trust CCRPS-certified professionals because the training reflects real CRA job expectations, not just theoretical knowledge. The certification can be listed on LinkedIn, resumes, and CVs to validate your CRA exam readiness and trial oversight competence.
Final Thoughts
Becoming a certified Clinical Research Associate isn’t just about passing an exam—it’s about mastering the regulatory mindset, monitoring precision, and ethical responsibility required to oversee complex clinical trials. The CRA exam is designed to test not only your knowledge of guidelines, but your ability to interpret, apply, and act under real-world pressures.
By following a focused plan—starting with exam structure awareness, deep-diving into core regulatory areas, using mock exams, and aligning your prep with industry-approved resources—you position yourself for both exam success and career advancement. Whether you're transitioning into the CRA role or strengthening your current path, your preparation reflects your readiness to lead site oversight with compliance and confidence.
| 📊 What’s your biggest challenge in preparing for the CRA exam? | |
|---|---|
| 🔘 | Understanding regulatory guidelines like ICH-GCP and FDA |
| 🔘 | Applying knowledge to real-world case scenarios |
| 🔘 | Sticking to a consistent and strategic study schedule |