Clinical Research Associate vs. Clinical Research Coordinator (CRA vs. CRC)
When you start a new job, decide on a career path, or want to learn more about an industry, the job titles, acronyms, and industry-specific language can be confusing!
This certainly holds true in Clinical Research, where job titles are often abbreviated into acronyms like CRA, CRC, CTM, CTA, PI, Sub-I, DM, and more—making it overwhelming for newcomers.
Clinical research is at the heart of medical advancements, and two key roles in this field are the Clinical Research Associate (CRA) and the Clinical Research Coordinator (CRC). While both contribute to clinical trials, their responsibilities, career paths, salaries, and industry demand differ significantly.
Suppose you’re considering a career in clinical research or looking to transition between roles. In that case, this guide provides an in-depth comparison, career insights, and industry trends to help you make an informed decision.
Clinical Research Associate vs Coordinator
What is a Clinical Research Associate (CRA)?
A Clinical Research Associate (CRA) is a healthcare professional responsible for monitoring clinical trials and ensuring compliance with regulatory requirements, ethical standards, and study protocols. They act as a liaison between the study sponsor (such as a pharmaceutical company) and the clinical research sites (hospitals or clinics). CRAs ensure trials follow established protocols, prioritize patient safety, and monitor data integrity throughout the study process.
A Clinical Research Associate (CRA), also called a clinical monitor or trial monitor, is a research professional with a minimum of a bachelor’s degree (often in nursing or life sciences). They may work under contracts or be hired by sponsors, Clinical Research Organizations (CROs), or freelancers by biopharma firms and research institutes.
CRAs play a critical role in ensuring that clinical trials comply with Good Clinical Practice (GCP) guidelines and all regulatory requirements. Unlike Clinical Research Coordinators (CRCs), CRAs frequently visit multiple research sites instead of being stationed at one location.
Required Skills for a CRA
A Clinical Research Associate (CRA) plays a crucial role in overseeing clinical trials and ensuring compliance with regulatory guidelines. To excel in this profession, a CRA must possess a combination of technical knowledge, organizational abilities, and interpersonal skills. Below are the key skills required for a CRA:
Strong understanding of regulatory guidelines such as FDA (Food and Drug Administration), EMA (European Medicines Agency), GCP (Good Clinical Practice), and ICH (International Council for Harmonisation).
Ensuring that clinical trials comply with ethical and safety standards.
Ability to handle multiple trials and regulatory requirements simultaneously.
Strong time management and multitasking skills to meet project deadlines.
Excellent communication skills to liaise between sponsors, investigators, and regulatory bodies.
Ability to work independently and as part of a team to ensure smooth trial operations.
Attention to detail to monitor trial progress and ensure data integrity.
Problem-solving and critical-thinking skills to address unexpected challenges during trials.
Proficiency in clinical trial software and documentation tools to manage electronic case reports and trial data.
Familiarity with data management systems for effective reporting.
Ability to travel frequently to various clinical trial sites for monitoring visits and audits.
Adaptability to different trial environments and regulatory frameworks.
Key Responsibilities of a CRA
A Clinical Research Associate (CRA) plays a crucial role in the management and monitoring of clinical trials, ensuring compliance with regulatory guidelines and ethical standards. CRAs work across various industries, including pharmaceuticals, biotechnology, medical devices, and academic research organizations.
Performing site selection, initiation, routine monitoring, and close-out visits to ensure smooth trial execution.
Ensuring protocol adherence and compliance with regulatory requirements.
Monitoring trial activities to ensure compliance with ICH-GCP (International Council for Harmonisation - Good Clinical Practice), FDA regulations, and specific study protocols.
Ensuring clinical research sites follow Standard Operating Procedures (SOPs) and ethical guidelines.
Tracking and reporting adverse events (AEs) and serious adverse events (SAEs) to regulatory bodies.
Ensuring patient safety throughout the trial process.
Reviewing Case Report Forms (CRFs) and verifying that all collected data is accurate, complete, and consistent.
Managing clinical trial data in accordance with Good Documentation Practices (GDP).
Acting as a liaison between sponsors, Clinical Research Organizations (CROs), and clinical trial sites.
Communicating with investigators and site staff to resolve protocol deviations and trial execution issues.
Conducting site audits to ensure proper documentation and data accuracy.
Identifying and mitigating risks to maintain trial integrity.
Preparing final clinical trial reports summarizing findings, compliance, and study outcomes.
Contributing to the identification of the benefits and risks of investigational drugs or medical devices.
Roles of a CRA
What is a Clinical Research Coordinator (CRC)?
A Clinical Research Coordinator (CRC) works at a single site under a Principal Investigator (PI) to manage day-to-day trial operations. They play a hands-on role in patient interaction, recruitment, and study execution. CRCs are important in ensuring smooth clinical trial operations at the site level and maintaining compliance with protocols. A Clinical Research Coordinator works within hospitals, universities, or private clinics, managing the day-to-day operations of clinical trials. Their primary focus is ensuring studies are conducted smoothly and according to protocol.
Required Skills for a CRC
A Clinical Research Coordinator (CRC) requires a diverse skill set to successfully manage clinical trials, including excellent communication, strong attention to detail, thorough knowledge of regulatory guidelines, and data management proficiency.
Strong interpersonal and communication skills for patient interactions
Understanding of clinical trial documentation and compliance
Ability to multitask and coordinate between stakeholders
Attention to detail for accurate data collection
Knowledge of GCP and regulatory requirements
Proficiency in clinical trial management software
Key Responsibilities of a CRC
A Clinical Research Coordinator (CRC) is primarily responsible for recruiting and screening potential study participants, managing informed consent processes, collecting and managing study data, coordinating site visits, ensuring proper medication storage and administration, and monitoring patient follow-ups and adverse events. CRCs maintain compliance with study protocols and regulations at the site level.
Managing study protocols and regulatory compliance at the site level
Recruiting and screening patients
Conducting informed consent processes
Collecting and managing study data
Coordinating site visits and audits
Ensuring proper medication storage and administration
Managing patient follow-ups and adverse events
CRA vs. CRC – The Core Differences
| Feature | Clinical Research Associate (CRA) | Clinical Research Coordinator (CRC) |
|---|---|---|
| Workplace | Travels between multiple trial sites | Works at a single clinical site |
| Primary Role | Monitors and audits trials | Manages trial execution and data collection |
| Interaction | Works with multiple sites and regulatory bodies | Works directly with patients and investigators |
| Salary (2024) | $85,000 – $120,000 | $55,000 – $75,000 |
| Growth Path | Can advance to Lead CRA or Clinical Trial Manager | Can transition to CRA or site manager |
| Education | Typically a Bachelor’s or Master’s degree | Bachelor’s degree, often in nursing or life sciences |
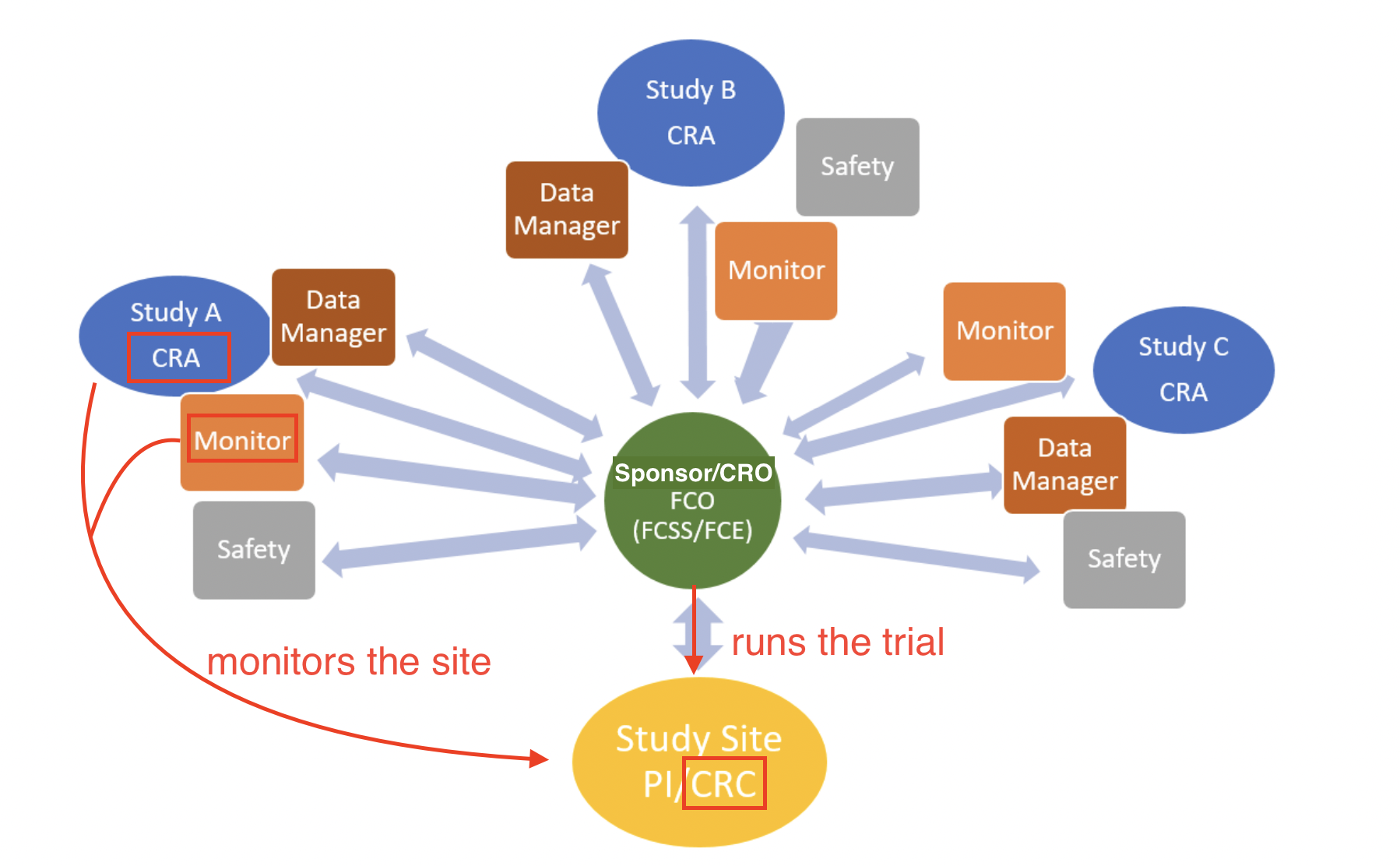
A CRA ensures compliance with ICH GCP and the clinical trial protocol by checking clinical site activities, making on-site visits (selection, initiation, routine, and close-out visits), verifying Case Report Forms (CRFs) against medical records, and communicating with the site’s CRC.
CRAs protect the ethical safety of human subjects and ensure the scientific integrity of the data collected through these processes.
Who Makes Up the Research Team?
Clinical Research Associate vs Coordinator Salary Comparison
Salaries for CRAs and CRCs vary based on experience, location, and employer.
CRAs tend to earn higher salaries due to the increased responsibilities and travel requirements. According to industry data, entry-level CRAs earn around $60,000 to $75,000 per year, with senior CRAs making $90,000 to $120,000+ annually.
CRCs earn slightly less, with salaries ranging from $45,000 to $65,000 at entry-level and reaching $70,000 to $90,000 for experienced coordinators.
Because CRAs manage multiple trial sites at one time, have at least a bachelor’s degree, and contribute to cost-effective trial outcomes, they usually earn more than CRCs. However, CRCs take on crucial responsibilities at the site level and are the backbone of a successful trial.
In 2025, the demand for clinical research professionals is increasing, and CRCs looking for career advancement can transition into CRA roles through certifications and specialized training programs. Salaries for CRAs and CRCs vary based on experience, location, and employer.
List of Relevant Courses:
Clinical Research Coordinator Certification: Enroll Here
Pharmacovigilance Certification: Enroll Here
Clinical Research Associate (CRA) Certification: Enroll Here
ICH-GCP Certification: Enroll Here
Clinical Trials Assistant Training: Enroll Here
Advanced Clinical Research Project Manager Certification: Enroll Here
Advanced Principal Investigator Physician Certification: Enroll Here
Medical Monitor Certification: Enroll Here
CRC vs CRA: CRC responsibilities include writing the IRB/Ethics Committee application (specific to each site unless trial is under a single IRB/sIRB), making/performing informed consent (IC) process, developing a budget for the site, subject recruitment, patient care, adverse event reporting (a CRA simply audits and ensures that no AEs were missed!), preparing the case report form (CRF) for the CRA to review against medical records, and submitting tons of data and records to the CRA/Sponsor at each site visit.
Site (CRC) vs. Sponsor (CRA)
Career Path and Growth Opportunities
Both CRAs and CRCs have distinct career paths:
CRA Career Path: Many CRAs start as CRCs before transitioning to monitoring roles. With experience, they can progress to senior CRA positions, clinical trial managers, or directors of clinical research. Some may also move into regulatory affairs or medical writing.
CRC Career Path: CRCs typically begin at entry-level positions and can advance to lead coordinator roles, clinical trial managers, or even principal investigators if they have a medical background. Some CRCs also transition to CRA roles for greater mobility and career progression.
Industry Demand and Job Outlook
The demand for both CRAs and CRCs is growing as clinical trials increase globally. The rise in pharmaceutical research, biotechnology advancements, and the need for regulatory compliance drive job opportunities in these roles.
CRAs are in particularly high demand due to the increasing complexity of clinical trials and regulatory oversight.
CRCs remain essential for conducting trials, especially in academic and hospital settings where patient interaction is critical.
Which Role is Right for You?
Choosing between a CRA and CRC role depends on your interests and career goals:
If you enjoy traveling, auditing, and ensuring regulatory compliance, a CRA role may be a better fit.
If you prefer working directly with patients, managing clinical trial operations, and coordinating research teams, a CRC position might be ideal.
“Starting as a CRC was the best decision I made. I learned the ins and outs of trial management at the site level, which made transitioning to a CRA role seamless. Within five years, my salary doubled.”
How to Become a CRA or CRC
Starting a career as a Clinical Research Associate (CRA) or Clinical Research Coordinator (CRC) requires the right mix of education, training, and experience. Below is a step-by-step guide to help you enter either role:
Becoming a Clinical Research Associate (CRA)
Earn a Bachelor’s Degree
A degree in life sciences, pharmacy, nursing, or health-related fields is typically required.
Gain Relevant Experience
Many CRAs start as CRCs, clinical trial assistants, or data managers before transitioning to a CRA role.
Obtain a CRA Certification (Optional but Beneficial)
Certifications like Certified Clinical Research Associate (CCRA) from ACRP or training from CCRPS can help you stand out.
Apply for CRA Jobs
Pharmaceutical companies, Contract Research Organizations (CROs), and biotech firms often hire CRAs.
Becoming a Clinical Research Coordinator (CRC)
Earn a Relevant Degree
A bachelor’s degree in life sciences, nursing, pharmacy, or a related field is preferred, though some roles accept an associate degree or relevant experience.
Gain Clinical Research Experience
Many CRCs start in entry-level research roles, such as research assistants, to learn about clinical trials.
Get Certified (Optional but Recommended)
Certifications like Certified Clinical Research Coordinator (CCRC) from ACRP or Certified Clinical Research Professional (CCRP) from SOCRA can improve job prospects and salary potential.
Apply for CRC Positions
Look for openings at hospitals, research institutions, universities, or pharmaceutical companies.
Tips for Success in Either Role
Stay updated on Good Clinical Practice (GCP) guidelines and industry regulations.
Gain hands-on experience in clinical trials to improve your skills.
Network with professionals and join clinical research associations for job opportunities.
With the right qualifications and experience, you can build a rewarding career in clinical research and play a key role in advancing medical treatments.
10 Lesser-Known Facts About CRAs and CRCs
CRAs travel extensively – some spend 50-70% of their time on the road.
CRCs handle 80% of patient interactions in clinical trials.
More than 50% of CRAs started as CRCs before transitioning.
The highest-paid CRAs work in oncology and rare disease trials.
CRAs often work remotely when not conducting site visits.
CRCs play a major role in adverse event reporting and patient safety.
CRA certification can increase salary potential by up to 25%.
CRCs often prepare reports that directly impact FDA approvals.
Both roles require strong knowledge of FDA, EMA, and GCP regulations.
CROs employ over 70% of CRAs, while hospitals employ most CRCs.
CRA vs. CRC: Which Career is Right for You?
Choosing between a Clinical Research Coordinator (CRC) and a Clinical Research Associate (CRA) depends on your skills, interests, and career goals. Here's a simple breakdown to help you decide:
A CRA Role Might Be Right for You If:
✔️ You like traveling between multiple research sites and working remotely.
✔️ You enjoy monitoring trials for accuracy, ensuring protocols are followed, and verifying regulatory compliance.
✔️ You have strong attention to detail and prefer working with data and documentation rather than direct patient care.
✔️ You want higher earning potential, as CRAs typically earn more than CRCs.
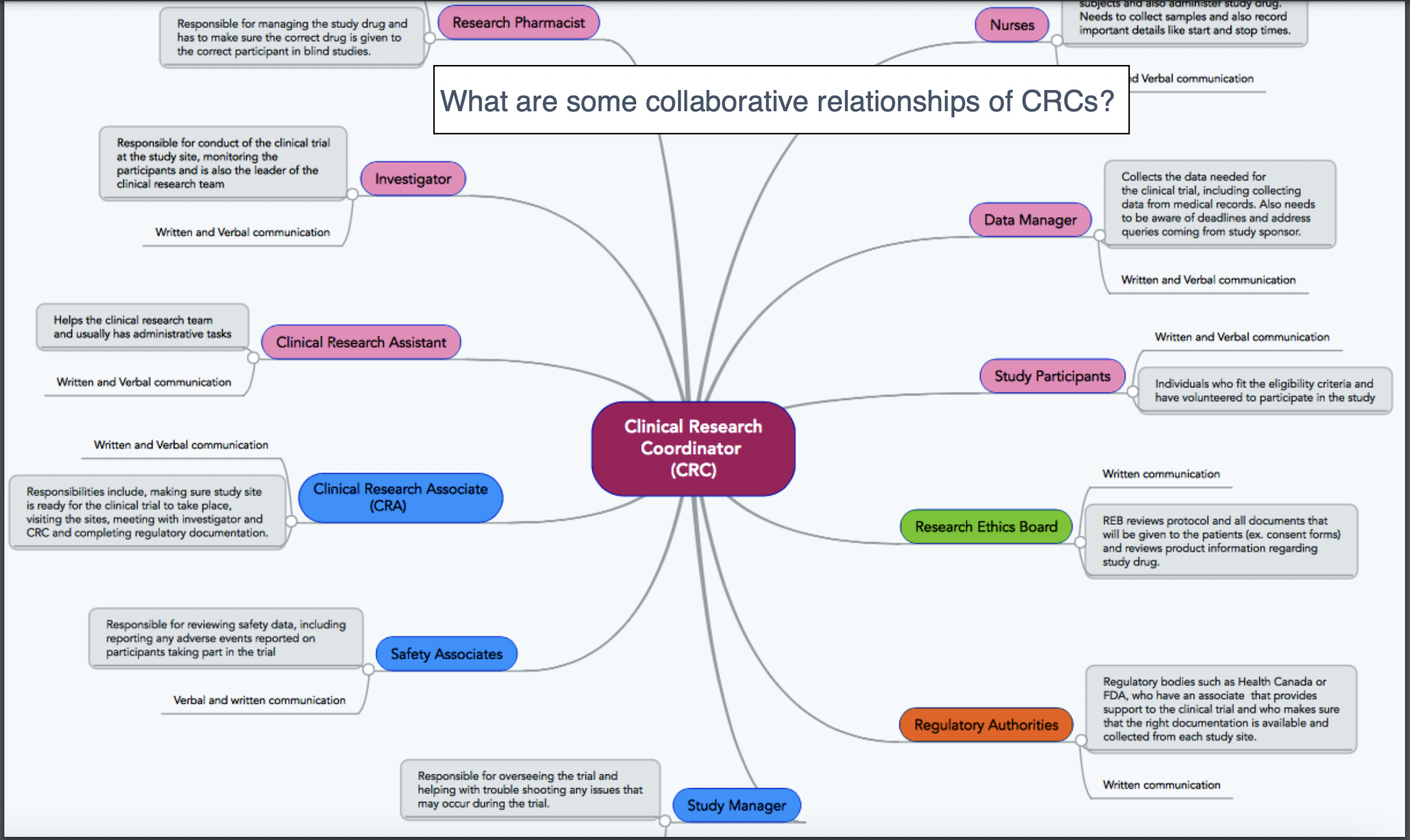
CRA interactions with other fields
A CRC Role Might Be Right for You If:
✔️ You enjoy working directly with patients and healthcare professionals.
✔️ You like managing day-to-day trial activities, such as patient recruitment, data collection, and site coordination.
✔️ You prefer a stable, site-based position at hospitals, clinics, or research centers.
✔️ You have strong organizational and multitasking skills to oversee trial logistics.
CRC interactions with other fields
Both roles are essential to the success of clinical trials, and your decision should align with your strengths and career aspirations. Whether you choose to be a CRC or CRA, clinical research offers exciting opportunities for growth and impact in the medical field.
CRC to CRA Bridge Program
For Clinical Research Coordinators (CRCs) aiming to advance into a Clinical Research Associate (CRA) role, structured training programs and certifications can significantly smooth the transition.
Certifications from organizations such as CCRPS, ACRP, and SOCRA provide the necessary knowledge and credentials to strengthen your application. Many CRAs start as CRCs, gaining hands-on experience in trial management, regulatory compliance, and data collection before moving into monitoring roles.
Typically, a certified CRC with at least two years of experience can transition to a CRA role within 6 to 12 months by enrolling in specialized training programs. These programs focus on Good Clinical Practice (GCP) guidelines, site monitoring, regulatory compliance, and sponsor interactions, preparing CRCs for the broader responsibilities of a CRA position.
Additionally, networking with industry professionals, gaining exposure to monitoring tasks, and seeking mentorship from experienced CRAs can further accelerate the transition. You can bridge into being a CRA within your own company or apply for CRA jobs by completing CRA certification and gaining experience with any on-site in-house CRAs your site may have.
CCRPS Clinical Research Certification provides advanced “senior”-level CRA certification for CRCs so that:
On resumes, you can prove knowledge competency of CRA tasks up to an advanced level (making job promotions easier).
During interviews, you can demonstrate practical application of CRA knowledge.
On the job, you can be efficient and diligent in preventing errors.
The Future of Clinical Research: Trends for 2025 and Beyond
The clinical research industry is evolving rapidly, with new technologies and regulations shaping how trials are conducted. As the clinical research industry evolves, professionals must stay updated with CCRPS emerging industry trends:
3. Stricter Regulatory Compliance
With increasing complexity in clinical research, 70% of companies now require CRAs and CRCs to have certifications from organizations like ACRP, SOCRA, or CCRPS.
Staying compliant with global regulations is becoming more critical than ever.
4. Growing Demand for Clinical Research Associates (CRAs)
The need for qualified CRAs is rising, with job growth expected at 15% annually.
More companies are investing in remote monitoring and virtual site visits, making CRAs a key part of clinical trials.
1. Rise of Decentralized Clinical Trials (DCTs)
By 2025, over 60% of clinical trials will follow a hybrid or decentralized model, allowing participants to take part from home instead of visiting a research site.
This shift improves patient access, reduces dropout rates, and speeds up trial completion.
2. AI and Machine Learning in Clinical Research
Artificial intelligence (AI) is transforming trial monitoring, helping detect errors and inconsistencies faster.
By 2025, AI-driven trial monitoring is expected to reduce data errors by 30%, making research more reliable and efficient.
The future of clinical research is bright, but staying ahead requires continuous learning, certification, and adaptability. Whether you're starting in clinical research or looking to advance, understanding these trends can help you build a successful and future-proof career.
“With AI-powered monitoring and virtual trials becoming the norm, CRAs need to develop strong technical skills. Those who adapt to these changes will lead the field in the next decade.”
Choose the Right Path in Clinical Research
Understanding the differences between Clinical Research Associates and Clinical Research Coordinators is essential for professionals looking to build careers in clinical research. While CRCs are the backbone of clinical trial sites, CRAs play a crucial role in maintaining the integrity of multi-site clinical trials. Clinical research is growing fast, and there’s a strong need for skilled professionals. Whether you decide to become a Clinical Research Coordinator (CRC) or a Clinical Research Associate (CRA), the right education, certification, and experience will help you succeed.
For CRCs aiming to transition into a CRA role, certifications and training programs offer the necessary credentials for career advancement in 2025. Staying updated with clinical trial industry trends and regulatory advancements can help secure high-paying roles and shape the future of medical research.
If you’re just starting out or looking to grow in your career, now is a great time to invest in training and certification programs. Keeping up with industry changes will open up more opportunities and help you build a stable future in 2025 and beyond.
Which Role is Right for You?
If you like working with patients, handling the day-to-day tasks of a clinical trial, and keeping things organized, a CRC role may be the right choice.
If you prefer overseeing research sites, making sure studies follow the rules, and traveling to different locations, a CRA career might be a better fit.
Both jobs are important in clinical research and offer great career growth. Knowing the differences can help you decide which path suits you best.
Take the Next Step in Your Clinical Research Career
📌 Check Out CRA & CRC Certification Programs at CCRPS
📌 Learn More About Career Opportunities in Clinical Research
With the right training and experience, you can build a fulfilling career that makes a real impact in medical research and helps improve lives.
For more insights on advancing your clinical research career, check out the A Full Guide on Becoming A CRA in 2025.