Clinical Research Courses - CTA vs. CRC vs. CRA
Clinical research is one of the most exciting fields in healthcare, providing an infinite amount of opportunities to actually make a positive difference in people’s lives, as well as building a fulfilling and rewarding career. Whether you are just starting your path as a Clinical Trial Assistant (CTA), or are getting ready to move up the ladder to a Clinical Research Associate (CRA) or a very important post of a Clinical Research Coordinator (CRC), the key to success lies in the knowledge of the qualifications, experience and training that is required for each position.
What Clinical Research Career and Course Is Best for You? Take Career Quiz
Start Here: Is CTA, CRA, or CRC Right for You?
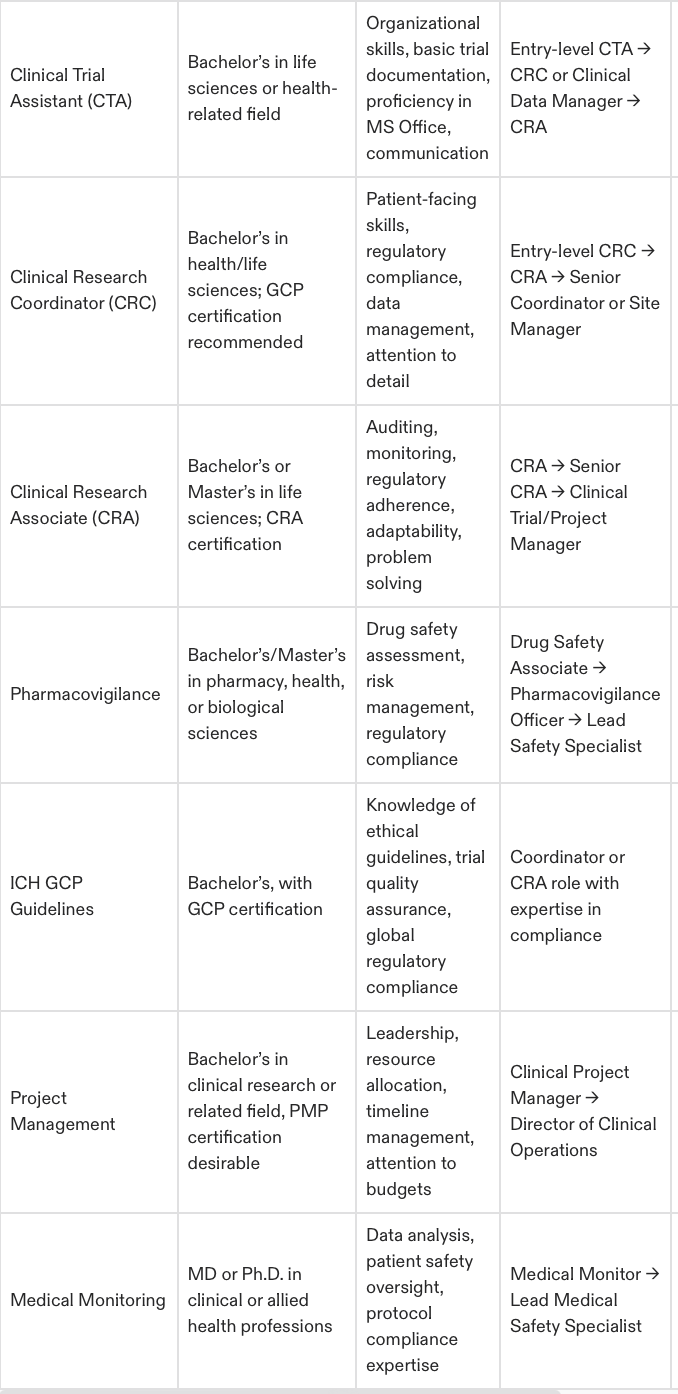
1. Clinical Trial Assistant (CTA)
CTAs are essential to clinical trials, executing all the non-clinical, operational tasks that need doing. They are the bedrock of trial documentation and site communication efforts, and so are a key part of the research process.
Background & Education
Ideal Background: Perfect organizational skills and great attention to details. Most of the CTAs are from the administrative, healthcare or life sciences background.
Education Required: The majority of employers seek a bachelor’s degree in life sciences or health sciences. Without a degree, good healthcare or laboratory administrative experience may suffice.
Experience and Career Path
You don’t have to have experience to start as a CTA. On the job training is provided to many CTAs which gives them a basic understanding of clinical trial operations.
Career Advancements:
Begin as a CTA handling trial documentation and logistics during the early stages.
Move into roles such as Clinical Research Coordinator (CRC) or Clinical Data Manager.
Progress to CRA positions with more training and experience.
CTA to CRC – The Logical Career Step Forward
For CTAs looking to move up, becoming a CRC provides a better income, more accountability and actual involvement in clinical trials. The CCRPS CRC Course can help make this transition within weeks.
2. Clinical Research Coordinator (CRC)
CRCs are an integral part of clinical trials, orchestrating many of the tasks that need to be done seamlessly at the site level. They control patient entry, records, and safety issues, and are in direct contact with investigators and patients. The role is more engaging within clinical research and can be considered as a stepping stone to intermediate level positions like CRA.
Background & Education
Ideal Background: Healthcare, nursing or administrative candidates are a usual transition from CRC positions.
Education Required: Generally a bachelor's degree in life sciences, health sciences or related fields is required. You will also stand out with training in Good Clinical Practice (GCP) and human subjects protection.
Experience and Career Path
Depends on the institution, but it usually helps to have some clinical administration experience or at least exposure to patient care. Most CRCs were once CTAs, or other healthcare workers who were able to use their training to get that job.
Career Advancements:
Begin as a CTA or similar entry-level role.
To become a CRC, get certifications similar to those offered by CCRPS. Transition into a CRC role with certifications like those offered by CCRPS.
For leadership or CRA roles — with site management experience, progress.
3. Clinical Research Associate (CRA)
CRAs are one of the most important and honored members of the clinical research team. They are there to monitor clinical trials, make sure they are complying with various regulatory standards, and helping to verify data accuracy as well as patient safety.
Background & Education
Ideal Background: Many CRAs are former CRCs or healthcare workers who have moved into the monitoring roles.
Education Required: A bachelor’s or master’s degree in life sciences, health sciences or related fields is required. Advanced degrees (e.g., PhDs) are also quite coveted, particularly in specialized therapeutic areas.
Experience and Career Path
CRA positions usually demand 1 - 3 years' experience in clinical trial coordination, site management or monitoring. Most professionals move up from the CRC position or gain specialized CRA training.
Career Advancements:
Start as a CRC, gaining site-specific knowledge.
Working in CRA monitoring roles? Get full training.
Further your career as a Clinical Trial Manager (CTM) or Director of Clinical Operations.
Career Comparison
Clinical Research Courses
Why Clinical Research Training Matters
Clinical research is crucial in closing the gap between medical innovation and patient care and it is important to ensure that new treatments are safe and effective. If you want to start a career in this field, first make sure to choose the right training. So if you want to work as a Clinical Trial Assistant (CTA), Clinical Research Coordinator (CRC) or a Clinical Research Associate (CRA), the course you choose will determine the knowledge and skills you gain and the path your career will take.
This guide offers an in-depth approach to selecting the best clinical research course, including practical tips and an overview of why CCRPS is a trusted leader in clinical research training.
Factors to Consider When Selecting a Clinical Research Course
To identify the program that is most relevant to your needs when assessing clinical research courses, concentrate on the following critical aspects.
1. Course Content That Matches Your Goals
A robust curriculum is the backbone of a reliable clinical research course. Assess whether the program includes the following key topics:
Good Clinical Practices (GCP)
Protocol development and trial management
Patient safety, informed consent processes, and Adverse Event (AE/SAE) reporting
Regulatory compliance and global standards (e.g., FDA, EMA)
Trial documentation, data capture, and site management
For advanced roles like CRC or CRA, look for specialized modules. For example, CCRPS provides separate programs tailored to the unique requirements of CRCs, CRAs, and CTAs, ensuring you’re prepared for the specific challenges of your role.
Tip: Request a syllabus or detailed breakdown of the course content before you enroll to verify alignment with your career path.
2. Accreditation and Industry Recognition
A course’s accreditation speaks volumes about its quality and credibility. Choose programs endorsed by reputable organizations or recognized by employers in the clinical research field. Accreditation ensures that the course meets rigorous standards and carries weight with hiring managers.
CCRPS courses are widely accepted across the industry and designed to meet global standards, giving graduates an advantage in their job search.
3. Flexibility and Accessibility
Your training shouldn’t interfere with your current commitments. Look for courses that offer:
Online learning for remote accessibility.
Self-paced schedules to accommodate your busy life.
User-friendly platforms that allow you to study from any device.
CCRPS courses are 100% online, self-paced, and accessible 24/7, allowing you to learn whenever it’s convenient for you.
4. Cost and Affordability
While education is essential, staying within budget matters. Evaluate the total cost of the course, including hidden fees, materials, and certification. A program should strike a balance between cost-effectiveness and quality of education.
CCRPS is renowned for offering high-quality courses at competitive prices while ensuring transparency in pricing. You get exceptional value without compromising on content.
5. Practical Learning Opportunities
While theory forms the foundation, practical application of knowledge is key to excelling in clinical research roles. Opt for courses that emphasize hands-on learning through:
Case studies and real-world examples.
Exposure to tools like Clinical Trial Management Systems (CTMS).
Scenarios that mimic clinical trial environments.
CCRPS incorporates practical, job-ready learning components to give students a real sense of what working in clinical research entails.
6. Career Outcomes and Employer Connections
Ultimately, your course should prepare you for the next step in your career. Evaluate the program's success in helping graduates secure roles. Check:
Job placement statistics.
Graduate feedback and testimonials.
Resources for resume building and career support.
CCRPS boasts a 90%+ job placement success rate and provides tailored resources like career counseling and employer-recognized certifications to set you apart.
7. Provider Reputation
Look for course providers with a proven track record of training success. Trusted providers, like CCRPS, not only offer excellent content but also provide ongoing support and maintain their standing within the clinical research industry.
Tip: Use reviews, ratings, and alumni testimonials to get a sense of how graduates feel about the program and its real-world application.
Why CCRPS is the Best Choice for Clinical Research Training
At CCRPS, we are committed to helping you meet your clinical research training needs with easily accessible, all-encompassing, and industry-standard courses that will help you move forward in your clinical research career.
1. Specialized Curriculum to Match Career Goals
CCRPS offers targeted CTA, CRC, and CRA courses that ensure you train specifically for your desired role. Their curriculum is industry-designed and regularly updated to meet employer standards.
2. Flexible and Fully Online Training
With CCRPS, you can study on your own schedule, anywhere in the world. This accessibility means professionals with busy lives don’t have to sacrifice convenience for quality.
3. Proven Career Placement Success
CCRPS certifications are trusted by sponsors, CROs, and research institutions globally. Most graduates report significant career advancement or job offers shortly after completing their training.
4. Affordable Pricing with Excellent Value
CCRPS strives to make quality education affordable. You’ll gain access to industry-standard training without overspending.
5. Practical Learning for Real-World Success
CCRPS blends theory with hands-on learning through case studies and real-world scenarios designed to mirror clinical trial challenges.
6. Career Support
From resume reviews to personalized career counseling, CCRPS ensures graduates feel prepared to enter the workforce with confidence.
Final Checklist to Choose the Right Course
Before making your decision, answer these questions using the information provided by the course provider:
Does the curriculum meet my career goals?
Is the course accredited or widely recognized?
Is the course flexible and accessible for my schedule?
Is the pricing clear and affordable?
Do graduates have proven success in advancing their careers?
If the answer to all these questions is "yes," you’re on the right track. CCRPS ticks every box and has a proven record of producing industry-ready professionals.
Your Career in Clinical Research Starts Now
The clinical research industry offers incredible growth potential for professionals who invest in the right training. Whether you’re beginning your career as a CTA, transitioning to a CRC, or striving to become a CRA, CCRPS is here to help you achieve your goals.
Take control of your future today with CCRPS’s comprehensive training programs—your gateway to financial stability, career growth, and making a real difference in healthcare.
Enroll now and start building your clinical research career today:
-
A bachelor's degree in life sciences or health sciences is typically required, though relevant healthcare or laboratory administrative experience may suffice.
-
Gain experience in clinical trials, enhance your organizational skills, and consider obtaining certifications like those offered by CCRPS to make this career move.
-
A bachelor’s or master’s degree in life sciences or health sciences is necessary. Additional qualifications in clinical trial management can enhance job prospects.
-
GCP are international quality standards that govern clinical trials to ensure the safety of participants and the integrity of data. They are crucial for regulatory compliance and ethical conduct in research.
-
Yes, many reputable institutions, including CCRPS, offer comprehensive online courses that provide flexibility and accessibility for learners worldwide.
-
CRCs can progress to higher roles such as Clinical Research Associates or Clinical Trial Managers by gaining additional training and experience in site management and monitoring.
-
Look for course content that matches your career goals, accreditation, flexibility, affordability, practical learning opportunities, and positive career outcomes for graduates.
-
Certifications demonstrate your expertise and commitment to the field, making you a more attractive candidate to potential employers and can significantly enhance your career advancement opportunities.